Gold (Au) Element's Specifications
Gold is a rare precious metal known for as long as human history with a lot of applications in science, industry, jewelry, electronics, medicine and, etc. Elemental gold (Au) with the atomic number of 79 categorized in group 11 along with copper and silver.
Gold occurs naturally as a soft reddish yellow transition metal resistant against strong acids, oxidization and corrosion with a melting point of 1064˚C. It is also resistant to most bases except for potassium cyanide.Gold compounds’ chemical reactivity is attributed to the energy separation at HOMO and LUMO. This causes luminescence in many complexes of gold. Gold makes up 3×10-7% of Earth crust with the annual production of 3100 tones. Leading companies such as Nanografi contribute to many applications with a wide range of high quality and high performance gold nanoparticles.
Properties of Gold
Gold is among those few metals that are malleable. This means gold can be shaped like semi-transparent sheets, rolled or molded to any shape. It is also ductile which means it can be drawn out into thread or wire adequately small the thin to be employed in semiconductors. Gold is practically non-reactive element on the periodic table as it doesn’t engage in any reaction with oxygen therefore, it never bears corrosion or rust. However, gold corrodes only in aqua regia which is mixture of nitric and hydrochloric acids.
Gold is categorized among materials with the highest conductivity of electricity and heat and is widely used to produce long lasting electrical connectors in electronics and electrical appliances. The thermal conductivity of gold is 310 W m-1 K-1 at 20˚C with the electrical resistance of 0.02 µΩ. In addition, gold is a qualified material to reflect electromagnetic radiation such as ultraviolet, infrared and radio waves. This property makes gold a reliable metal to be applied in aerospace technology since the outer atmosphere and space is where there is a lot high energy radiation that can easily harm astronauts and destroy equipment. Due to the high compatibility of gold with the human biology and high antibacterial specifications, it is widely used in medicine and dentistry. In nano scales, however, gold exhibits considerably distinct behavior compared to its bulky form. Gold nanoparticles have found a lot of applications in science and technology which will be discussed in upcoming sections in detail.
Origin of Gold
All elements have been formed after the Big Bang and the process called fusion through which the nuclei of smaller elements fuse to form heavier elements. The fusion is powered by high temperatures at least 20,000,000˚C at the core of stars as well as high pressure. The Sun has enough power to generate elements lighter than Iron (Fe) but those heavier than that have their roots in other denser and much bigger stars than the Sun in other galaxies. Gold with the atomic mass of 196.9 Da is originated from supernovas and neutron stars and the collisions between them. Neutron stars are extremely dense and created when supermassive stars explode in a supernova as a spoonful of a neutron weighs as much as 5 billion tons on Earth. These explosions between neutron starts can send debris to space around one third the speed of light and can create gold as mush as the mass of Earth. Accordingly, the presence of gold on Earth is through meteorites that have collided our planet in during millions of years.
Structure and Color of Gold
Metallic gold has a crystalline structure a face-centered cub (FCC) which is responsible for its high ductility as well. The golden color of gold has been a mystery throughout the history with a secret that lies in this precious metal’s atomic and electronic structure. Although gold is a truly dense and heavy metal, the electrons orbit the nucleus so fast. This behavior can be explained using quantum mechanics and Einstein’s relativity simultaneously. Normally, metals reflect light in their pure metallic form, whereas gold absorbs the electromagnetic light with shorter wavelength and higher energy through electrons transition from the d-band to vacant orbital in the conduction band in its pure metallic form. This gives gold the color which is so sparkling and bright.
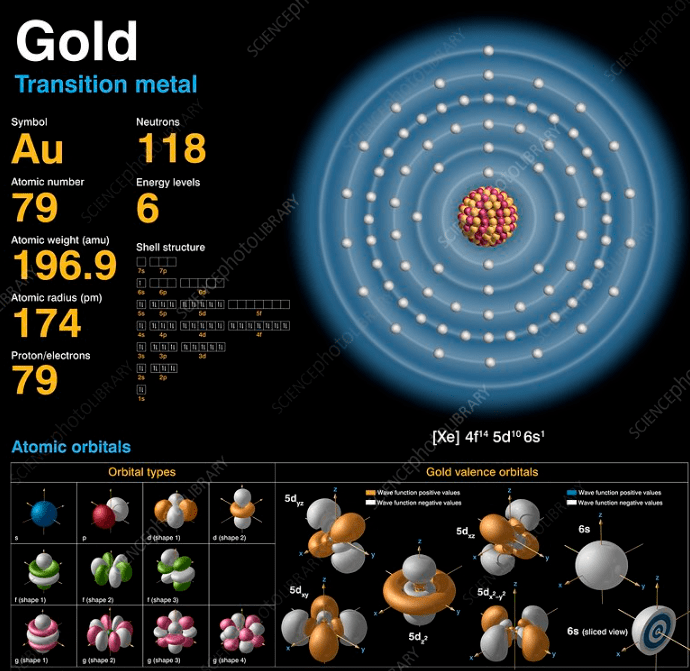
Figure 1. Atomic structure of gold.
Applications of Gold
Stability, inertness against a big directory of chemicals in extreme environments, physical properties, abundance and elegance of gold have all given this mysterious metal a huge value not only in science, technology and industry but in economy as a wealth code and a measure of economic growth.
Gold Compounds as Anticancer Agents
Gold compounds have been categorized as metallodrugs with health effects in cancer therapy. There many reports of gold compounds, Au (I) and Au (II), with antiproliferative properties against tumor cells making them potential pharmacological interest. The health effects of gold are due to its unique chemistry. In fact, the gold application for medical purposes isn’t limited to modern age as it was extensively used in early human history as an anti-infection and antitubercular agent.
To learn more about the use of nanoparticles in cancer treatment, visit our blog page.
Gold Nanoparticles
Gold nanoparticles (AuNPs) are regarded as a model system to explore and study a wide range of applications including biolabeling, electron transfer, DNA melting, crystal growth, catalysis and self-assembly due to its optical and electronic properties. When AuNPs are protected or stabilized by thiolated ligands, they exhibit staggering stability against aggregation and decomposition. Aggregation of as many as 300 gold nanoparticles appears to be different optically and electronically enabling them to serve as a capable chemical platform for desired activities.
Gold nanoparticles show catalytic behavior to oxidize CO heterogeneously at low-temperatures. Moreover, AuNPs adopt three different classes of electrochemical properties namely molecule-like voltammetry, bulk continuum voltammetry and quantized double-layer charging voltammetry. Gold nanoparticles (AuNPs) as ligand stabilized agents have drawn attention in biomedical applications. Unique properties of gold nanoparticles can be employed to synthesize new materials with functionality in biological environments.
Gold in Electronics Industry
Among the various industrial applications of gold, the electronics industry seems to have taken a lot of advantages of gold in manufacturing devices and gadgets. Since solid state electronic devices suffer corrosion at smaller voltages, gold is widely applied as a highly qualified conductor to get over this really deterrent problem. Therefore, electronics that are made of gold serve reliably and perfectly.
Did you know that graphene makes electronic devices lighter, faster and more durable? Learn now.
Gold in Aerospace chnology
Gold is a favorite and widely used metal in space devices and equipment. In addition to strong conducting ability, gold is employed to stabilize the extreme temperatures of the spacecraft and reflect infrared radiation as in the absence of gold coating, the darker parts of the satellites and spacecrafts can absorb too much heat and radiation. As it’s known, lubricants that are technically made of organic materials are used to facilitate to operation of mechanical parts of machines. However, organic compounds that are used for lubrication purposes are volatile and can easily be decomposed by the strong high frequency radiation in vacuum space. Therefore, using gold thin films comes in really useful as the layers of molecules slipping on each other makes it easy for the parts to move easily.
Have you heard about the new method for the production of gold nanoparticles? Learn now.
Conclusion
Gold has been a wonder material in human history and for as long as we know about the written history. It not only determines how wealthy a person or country with its gold reserves is, it has considerable impact in molecular levels. The world economy is directly dependent on the gold and the consequences of world policy with all the threats, attacks and conflicts first appear in the price of gold.
To explore the website of Nanografi, a leader in advanced materials, renowned for its extensive collaborations across more than 100 countries and offering a diverse product range exceeding 4000 items, you can visit their official site directly. This link will guide you to more detailed information about their innovative products and solutions: Visit Nanografi.
References
Fackler, J. P., Assefa, Z., Forward, J. M. & Grant, T. A. ELECTRONIC PROPERTIES OF GOLD ( I ) COMPOUNDS RELEVANCE TO CHEMICAL REACTIONS. 223–231
Gold, atomic structure - Stock Image - C018/3760 - Science Photo Library. (n.d.). Retrieved May 10, 2024, from https://www.sciencephoto.com/media/553941/view/gold-atomic-structure
Iron Nanoparticles: Properties and Applications - Nanografi Nano Technology. (n.d.). Retrieved May 10, 2024, from https://nanografi.com/blog/iron-nanoparticles-properties-and-applications/
Nobili, S. et al. Gold Compounds as AnticancerAgents : Chemistry , Cellular Pharmacology , and Preclinical Studies. 30, 550–580 (2009).
Sardar, R., Funston, A. M., Mulvaney, P. & Murray, R. W. Gold Nanoparticles : Past , Present , and Future †. 25, 13840–13851 (2009).
Using Nanoparticles for Cancer Treatments - Nanografi Nano Technology. (n.d.). Retrieved May 10, 2024, from https://nanografi.com/blog/using-nanoparticles-for-cancer-treatments/
Use of Graphene in Electronics - Nanografi Nano Technology. (n.d.). Retrieved May 10, 2024, from https://nanografi.com/blog/use-of-graphene-in-electronics/
Recent Posts
-
Why Not All Graphene Is the Same: Structural Differences That Define Performance
Introduction Graphene is one of the most widely discussed materials in advanced technology, yet its …23rd Jan 2026 -
What Are Advanced Materials and Why They Matter for High-Tech Industries
Introduction Advanced materials are no longer peripheral inputs in technology development; they have …9th Jan 2026 -
Art That Changes Over Time: Stimuli-Responsive Materials Enabled by Nanotechnology
Art, design, and advanced materials are converging at an unprecedented pace, with nanotechnology in …19th Dec 2025






