Understanding Metal Organic Frameworks - Nanografi Blog
Metal-organic structures are compounds formed from metal ions and organic molecules so that a hybrid material of one, two, or three dimensions is created. These hybrid structures can be porous or non-porous and offer multiple functionalities. Metal-organic structures Organometallic crystal frames (MOF) fall into the category of high potential porous materials, which can be used in gas storage, adsorption/separation, catalysis, as adsorbents, in magnetism, sensor design, and administration of drugs. In this article, we’ll discuss all the relevant aspects of Metal-Organic Frameworks.
Metal-organic structures (MOFs) are one of the most promising discoveries in materials science. In addition to having potential applications in the energy, biotechnology, and biological fields, these synthetic structures can be useful in the manufacture of intelligent drugs. There are a variety of methods for inducing drug release, such as the pH response, the magnetic response, the ionic response, the temperature and the pressure response. But for them to work optimally, two problems must be solved: the toxicity of metals and their instability (because MOFs tend to degrade in the environment). The latter is what scientists around the world are exploring in their latest research.
Check Our Other Article About Metal Organic Frameworks
Production of MOF
The sonochemical synthesis of MOF is a promising technique as metal-organic crystals are produced very efficiently and environmentally friendly. The ultrasound production of MOF can be linearly on a larger scale than the preparation of small samples in the laboratory for complete commercial production. MOFs are typically formed by self-assembly where secondary construction units (SBUs) connect with organic spacers (ligands) to create complex networks. Organic spacers or metallic SBUs can be modified in order to control the porosity of the MOF, which is crucial in terms of its functionalities and its usefulness for particular applications.
Sonochemistry MOF synthesis Ultrasonic irradiation and thereby caused cavitation are famous for their exceptional properties on chemical reactions, known as Sonochemistry. The ferocious collapse of cavitation bubbles produces hot spots with excessively high transient pressures, cooling speeds, and temperatures, as well as resulting liquid jets and shock waves. In this cavitation, hot spots, nucleation and crystal growth, for example by the maturation of Ostwald, is induced and endorsed. Though, the particle size is restricted as those hot spots are categorized by great rate of cooling which means that the reaction temperature is in the range of milliseconds. Ultrasound is known to synthesize MOF quickly under mild process conditions, such as solvent-free, at room temperature and under ambient pressure. Studies have shown that MOFs can be produced profitably at high performance by the sonochemical process. The Sonochemical process for MOF synthesis is a green and environmentally friendly method.
A New Metal-Organic Frame That Easily Separates Gases
Researchers have discovered a new type of microporous metal-organic framework (MOF) that shows exceptional separation properties in the production of polymer grade ethylene, an industrial chemical of great importance, although expensive.
According to this new study, the new MOF based on iron peroxide is the best material available for reports on the separation of ethane-ethylene and has great potential for industrial applications. Ethylene is a chemical of great application and importance in the manufacture of numerous products, such as polyethylene plastics. The study has been published in the journal Science.
However, the widely used current method to obtain this chemical requires its separation from ethane by a cryogenic distillation process, one of the processes with the highest energy consumption in the chemical industry.
The development of an efficient ethylene-ethane separation process in terms of costs and energy is highly anticipated and is among the most important to guarantee future energy-efficient industrial separation processes. Gas separation processes based on microporous materials, such as MOFs, have proven to be a promising alternative to traditional ethylene-ethane separation methods.
However, many of these materials preferably capture ethylene, which must then be separated from MOFs by additional processes. In addition, to achieve polymer grade purity levels (> 99.95%), multiple separation cycles are required, which limits their overall efficiency and profitability. Inspired by natural metalloenzymes that contain iron peroxide sites and preferentially bind alkanes such as ethane, Professors from the Taiyuan University of Technology and the University of Texas at San Antonio, and their colleagues have synthesized a similar MOF using Fe2(O2) and have evaluated their separation performance.
They noticed that the material presented a strong preference for the bonding of ethane over ethylene. In addition, the authors show their ability to produce polymeric grade ethylene (99.99%) from a single separation cycle under environmental conditions. The high yield of the ethane-ethylene separation was further validated by theoretical calculations, simulations, and experiments.
Mofs Extract Water from The Air, Even In Arid Areas
Researchers at the Massachusetts Institute of Technology (MIT) have produced a system that, at first glance, seems to get drinking water from nothing, but actually generates it from the air, and is even able to extract it from the driest desert air, which is good news for people who live in areas far from water sources and extremely arid places.
Scientists explain that, even in the driest places on Earth, there is always some moistness in the air, and a practical method to obtain that moisture could be crucial to surviving in dry places. The system is built on relatively new high-surface materials known as organic metal frames (MOFs), which can obtain drinking water from air from anywhere in the world with humidity as low as just 10%. The existing methods of obtaining water from air demands high levels, up to 100% humidity for fog collection techniques, and more than 50% for spray-based systems, which also need great amount of energy to cool.
The new device was tested on the roof of Arizona State University, in the city of Tempe, and the team showed that it is possible to collect water, even at dew points below zero. However, they said there is much work to be done to improve the process.
The device works only with sunlight because it has photovoltaic cells implemented. For now, little water is produced, but, in the next stage, the system will be expanded to produce 1/4 of a liter of water per day per kilogram of MOF. Scientists hope that, in the near future, several liters of water can be generated per day.
One of the goals for next year is to build a prototype system that can supply water to homes. The team examined the water obtained by its technology and results indicated no traces of any impurity. The mass spectrometer test showed that there is nothing in the MOF that leaks into the water. MOF is very steady and we can get high-quality water.
There are other technologies, such as WaterSeer, a machine that is planted two meters underground. At the top, it has a vertical wind turbine that rotates the blades of an internal fan to introduce ambient air into an underground chamber. Because the chamber is cooled by the surrounding earth, water condenses in a reservoir and it can be pumped outside to obtain drinking water. This invention promises to produce up to 37 liters of clean water in one day and can always work, even when there is no wind. The system is still in the testing phase and, like the MIT developments, it is expected that they will soon be able to start work, especially in arid areas and where water is scarce.
New Metal-Organic Structure for Electronic Applications
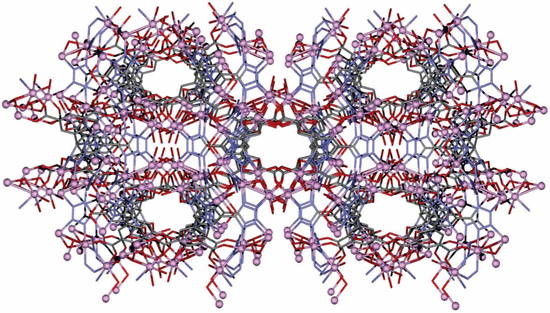
Scientists have unequivocally observed electronic transport of semiconductor type in a metal-organic structure for the first time. This unusual manners, together with profitable manufacturing, opens the way to employ metal-organic structures as active elements in electric devices.
Inorganic semiconductors such as germanium, gallium arsenide, silicon, are fundamental elements of recent electronics. They are presently used in a variety of devices, for example, LEDs, solar cells, and computer chips. The inorganic semiconductors are crystalline, pure and neat. These characteristics make them good conductors of energy under the use of an external voltage.
However, its high crystallinity and purity, which is achieved through very high-temperature processes, makes them very expensive elements to produce. A "low-cost" alternative is semiconductors based on organic elements, for example, polymers, which can be processed at low temperatures, even at room temperature. The problem with low-temperature production is that it is usually associated with the presence of defects in the crystalline network and the disorder of the samples. These defects strongly compromise the electrical properties of semiconductors based on organic materials, making most of them behave as insulators to the passage of current.
Scientists (IMDEA Nanoscience and MPI-P) have established and categorized a metal-organic structure (MOF), an organic material manufactured at ambient temperature that, extraordinarily, behaves like an inorganic semiconductor in terms of its electrical properties. These effects open the way to exploit MOFs as active elements of electronic devices.
The MOFs created to date, although they have a crystalline structure, have shown zero or very low electrical conductivity. This is mainly due to the insulating nature of the ligands that coordinate the metal centers. This new MOF, created by Prof. Feng's group in Dresden, is also orderly and highly crystalline solid, but gotten from conductive organic ligands, which are synchronized by metal nodes. The constituent elements of the crystal have been assembled to produce a semiconductor 2D material, in a process called self-assembly, at room temperature. This material is as fine as a single layer of atoms, a feature that facilitates the transport of electric current in the 2D plane.
The metals we know are hard because they are compact, they have structures that do not usually have too much distance between the centers of their molecules. However, different investigations have discovered that if these centers can be distanced using organic ligands, porous materials can be created that serve to store gases such as hydrogen. MOF has the appearance of a three-dimensional mesh with empty spaces. These spaces can be used to place a molecule. In MOF, zinc ions are linked to white (carbon) ligands. This structure houses the green spheres at its center, which become hydrogen molecules. This particularity has caused MOFs to be considered as compounds that serve to release various therapeutic drugs along with its other marvelous applications.
References
https://www.sciencedirect.com/science/article/abs/...
https://www.sciencedirect.com/topics/chemistry/met...
https://www.sciencedirect.com/science/article/pii/...
https://www.ncbi.nlm.nih.gov/pubmed/30361370
http://nanociencia.imdea.org/home-en/news/item/met...
https://www.researchgate.net/publication/325656051...
Recent Posts
-
Key Performance Metrics in Nanomaterials: How Performance Is Actually Evaluated
Introduction When nanomaterials are discussed, performance is often reduced isolated peak performanc …29th Jan 2026 -
Why Not All Graphene Is the Same: Structural Differences That Define Performance
Introduction Graphene is one of the most widely discussed materials in advanced technology, yet its …23rd Jan 2026 -
What Are Advanced Materials and Why They Matter for High-Tech Industries
Introduction Advanced materials are no longer peripheral inputs in technology development; they have …9th Jan 2026