Zinc Oxide CO2 Sensors: An Article Review
Carbon dioxide (CO2) sensors have an important position in our lives in many different application areas. Detection of CO2 makes possible to take precautions for unexpected cases such as fire or it can be used in air-quality and greenhouse-gas monitoring. Metal oxides are used sensors for these purposes. Researchers have been trying to reduce costs and size of these sensors because these parameters limit the usage of this sensor for some certain purposes. Also, metal oxide sensors show poor sensitivity at lower temperatures. Graphene and any other 2-D materials are also alternative materials for sensors, but they have some disadvantages such as being irreversible and requirement of long recovery time. Moreover, sensors that are working at room temperature are very sensitive to temperature variation and humidity changes which are not desirable, especially in the case of long response times. We have reviewed Indian Researchers Srinivasulu Kanaparthi and Shiv Govind Singh’s article “Chemiresistive Sensor Based on Zinc Oxide Nanoflakes for CO2 Detection” published in ACS Applied Nano Materials.
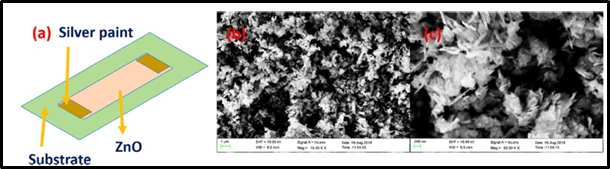
Figure 1: (a) Schematic of ZnO Chemiresistive gas sensor. (b and c) Morphology of ZnO Nanoflakes
Requirements for a perfect CO2 sensor are excellent sensitivity, fast response and good recovery times. Researchers, from Indian Institute of Technology, conducted an experiment by using Zinc Oxide (ZnO) nanoflakes for CO2 detection at low temperatures. As a result of this experiment, ZnO nanoflakes exhibited an excellent performance. They were very sensitive to CO2 due to having a large surface-to-volume ratio. Also, the response time was ultrafast. These two properties make ZnO nanoflakes a great alternative sensor for CO2 detection. However, energy consumption of these nanoflakes is relatively high compared to other sensors, but it is still a great alternative for cases in which high-temperature gas sensing is required. In addition, thanks to its cross-sensitive property to the other gases like NH3 or H2S, this sensor can be used in indoor or outdoor applications.
The CO2 gas sensing mechanism can be explained by oxygen vacancy principle. Chemiresistive behavior of the sensor can be determined by the oxygen vacancies, which act as electron donors, on the surface of the metal oxide. ZnO has high oxygen vacancy, so it behaves like a n-type semiconductor. Another advantage of ZnO nanoflakes is that its gas sensing behavior can be controlled by reoxidation or reduction of Zinc Oxide.
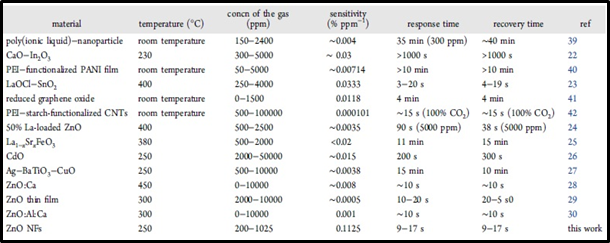
Table 1: Comparison of the ZnO Nanoflakes Sensor with Other Sensors Reported in the Literature
ZnO nanoflakes based sensor is compared with graphene, carbon nanotubes or 1-D metal oxides at similar gas concentration in terms of sensitivity and response time which play crucial role in determining a good sensor. As a result of this comparison, it was observed that the sensor in the experiment outperforms any other sensor. As can be understood from the Table 1, although the gas concentration of this sensor is relatively low compared to other sensors, it has higher sensitivity and faster response time. These properties are vital in many applications for the human health and safety.
Recent Posts
-
Nanotechnology in Medicine: How Nanomaterials Are Extending Human Life
The promise of nanotechnology in medicine is no longer hypothetical—it’s rapidly becomi …27th Jun 2025 -
Nanomaterials in AI Hardware: Building Smarter, Faster, and Brain-Inspired Systems
AI’s future depends not just on algorithms, but on the materials that bring intelligence to li …16th Jun 2025 -
An Overview of Laser-Induced Graphene: Methods, Properties, and Applications
A 3D porous substance known as laser-induced graphene (LIG) is developed by directly writing carbon …10th Jun 2025