What is Graphene: The Ultimate Guide (2025)
Graphene is a one-atom-thick sheet of carbon atoms arranged in a honeycomb (hexagonal) pattern.
Despite being just a single layer, it boasts 200 times the strength of steel and excellent electrical and thermal conductivity. First isolated by Andre Geim and Konstantin Novoselov in 2004, graphene’s potential has unleashed in the last two decades. This guide provides a comprehensive overview—from graphene’s discovery to its wide-ranging applications in electronics, energy, biomedicine, and more.
Recent 2025 findings in materials science underscore graphene’s growing influence in sustainable innovation—from advanced sensors to next-generation energy solutions. Thanks to ongoing research and industrial collaborations, graphene is on the brink of mainstream adoption, promising breakthroughs in electronics, battery, medicine, aerospace, and more.
Key Highlights
- Single-Atom Thickness, Extraordinary Strength: Up to 200× stronger than steel.
- High Electrical & Thermal Conductivity: Electrons move at near-light speeds on its surface.
- Lightweight & Flexible: Potential for bendable electronics, sensors, and protective materials.
- Broad Applications: From ultra-fast batteries to advanced biomedical devices.
- Evolving Production Methods: Chemical Vapor Deposition (CVD), mechanical exfoliation, and liquid-phase exfoliation are making large-scale graphene more accessible.
In this ultimate guide, you’ll explore graphene’s journey—from its Nobel Prize-winning discovery to the cutting-edge innovations shaping its future. Whether you’re curious about its properties, production methods, or real-world applications, this comprehensive resource will show you why graphene is often called the “material of the future.”
Table of Contents
Introduction: What Is Graphene? A Brief Look at the Literature
Graphene is a single-atom-thick layer of carbon atoms arranged in a two-dimensional hexagonal (honeycomb) lattice, distinguished by its exceptional mechanical, thermal, and electrical properties (Geim, 2009; Ferrari et al., 2015). It was first isolated in 2004 using a simple “Scotch tape” technique (Novoselov et al., 2004), an achievement that ultimately led to the 2010 Nobel Prize in Physics (Nobel Prize, 2010). Essentially, graphene is derived from graphite, the well-known carbon allotrope found in items like pencil leads and battery components—stacked graphene layers form bulk graphite.
When researchers peeled off a single atomic layer from graphite, they revealed a material boasting remarkable strength (up to 200 times stronger than steel) and extraordinary electron mobility (Lee et al., 2008; Neto et al., 2009). These unique 2D characteristics underlie graphene’s diverse applicability across electronics, energy storage, medicine, and advanced composites (Bonaccorso et al., 2012; Sun et al., 2021). As scalable production methods like chemical vapor deposition and liquid-phase exfoliation continue to advance, graphene’s impact on next-generation technologies grows increasingly promising (Park et al., 2014; Hernandez et al., 2008).
Graphene Structure
Graphene is an allotrope of carbon—just like graphite and diamond—consisting of a single layer of carbon atoms arranged in a hexagonal lattice. It is formed by hexagonal rings of carbon atoms, one of the most important and abundant elements in nature. Each layer of hexagonal graphene ring has a height of approximately one carbon atom and this characteristic, together with the application of very specialized techniques, allows us to obtain extraordinarily thin graphene layers.
The perfect graphene would contain only hexagonal rings although, in reality, pentagonal and heptagonal rings may appear that are considered irregularities and imperfections in the structure of graphene. This structure is the basis of other graphite substances such as fullerenes, carbon nanotubes or graphite itself.
Graphene is the basic elementary unit in 2D to build all the graphite materials of other dimensions. For example, it can be arched in zero-dimensional structures (0D), as is the case with fullerenes, it can be rolled up in 1D structures, giving rise to carbon nanotubes and, finally, it can be stacked successively giving rise to three-dimensional graphite (3D).
According to the IUPAC (International Union of Pure and Applied Chemistry), the term graphene should be used when talking about "reactions, structural relationships or other properties of individual layers" of carbon. Taking this into account, it is not correct to describe graphene as "layers of graphite" (graphite implies 3 dimensions while graphene implies carbon bonds in two directions), "carbon sheets" and similar concepts.Thus graphene can be defined as an infinitely alternating polycyclic aromatic hydrocarbon of six carbon atoms rings, that is, it a flat molecule composed of carbon atoms that form a pattern of hexagonal rings.
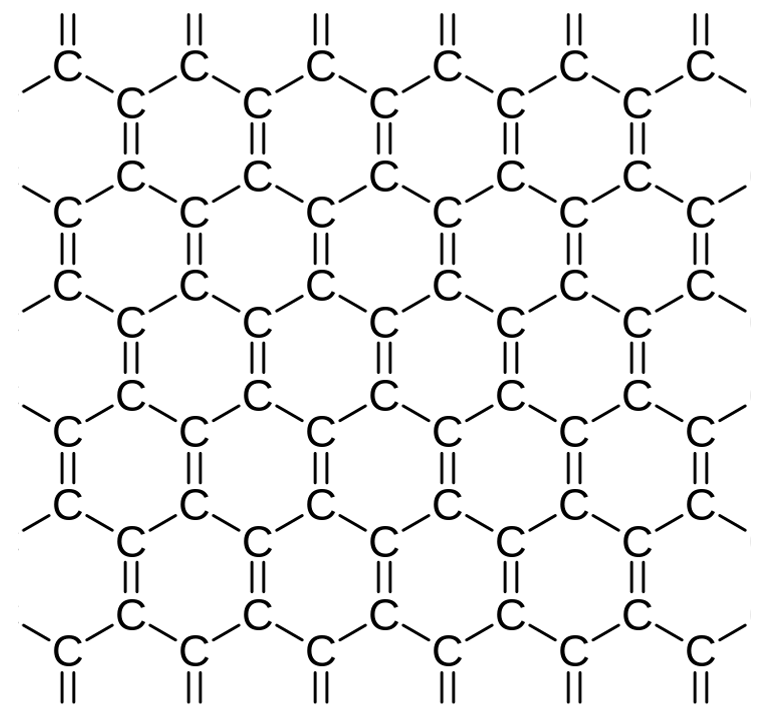
Figure 1. Graphene Structure
Part 1: History of Graphene
The 21st century, after the eras of coal and silicon, has been defined as the era of Graphene, considering that graphite, the allotrope of carbon from which Graphene originates, is a poor and abundant material in nature so it can be exploited on an industrial level. The carbon atom is one of the most intriguing elements of the entire periodic table, a brick on which the entire system of organic chemistry is built and above all constitutes the fundamental element of Graphene.
But where does the story begin? Very far away, even from the Middle Ages when graphite was used as a marking tool. In practice, when we rub a graphite sample on a sheet or on another support, very thin layers of material adhere to the support and then remain there. Around 1560, Bernacotti gave the idea of a lead pencil. In 1795, its production on a large scale came into existence. It is logical to think that an object as humble as a pencil may have contributed, in some way, to increase the ability to acquire culture even on the part of the less advantaged social strata that rarely could afford the luxury of buying books.
The history of Graphene is long and peculiar from many points of view, it is curious and partly surprising to see how. Despite the fact that graphite was in our hands for centuries, Graphene was never actually recognized as a material destined to change the entire history of science; this lack of recognition was probably due to the lack of adequate investigation tools. Now we can, with good reason, state that this innovative field of research is the most explored in the world, not only from an experimental point of view but also from a theoretical point of view.
The first work on the band structure of the single graphite plane date back to a period between 1947 and 1958, a time when, however, this material was considered only an academic exercise, because the same researchers believed that it was impossible to obtain strictly two-dimensional system that is simultaneously stable even in the isolated state. Boehm and his colleagues, in 1986, coined the term " graphene" combining the word graphite and a suffix that refers to a particular type of hydrocarbons, i.e. polycyclic aromatic hydrocarbons. In 1999, the first free graphite was built and only 5 years later, the history of science changed completely.
In 2004, at the University of Manchester, the scientists Geim and Novoselov, using a graphite sample and a simple adhesive tape discovered the material of the future that is Graphene. From a structural point of view, we can imagine Graphite as a book whose sheets are made of Graphene.
Figure 2. Nanografi's Graphene & Nanotechnology Journey
Discovery of Graphene
The discovery of graphene is relatively recent, and perhaps for this reason it remains unfamiliar to many people. In 2004, Andre Geim and Konstantin Novoselov from the University of Manchester had the unconventional idea of using ordinary adhesive tape (commonly known as Scotch tape) to peel off single layers from a piece of graphite. This simple yet ingenious technique led to the first isolation of graphene in history, an achievement that earned them the 2010 Nobel Prize in Physics (Novoselov et al., 2004; Nobel Prize, 2010).
This groundbreaking material—often called the “wonder material” of the 21st century”—is a crystalline allotrope of carbon, akin to diamond and graphite. In essence, diamond, graphite, and graphene are all composed of carbon atoms bonded in different configurations. Graphite itself consists of multiple carbon layers in a hexagonal lattice, whereas graphene represents just one of those layers. Although the discovery might sound like a curiosity for chemistry enthusiasts, graphene’s potential for commercial applications and profitability has turned out to be extraordinary. By 2025, over 3,000+ research papers on graphene had been published, with more than 200 companies and startups worldwide engaging in graphene-related research.
Who Discovered Graphene?
In 2004, Andre Geim and Konstantin Novoselov successfully isolated graphene—a single-atom-thick layer of carbon—from bulk graphite. This revolutionary finding showcased graphene’s unique and superior properties, from its remarkable mechanical strength to its exceptional electrical conductivity. Their discovery was recognized with the Nobel Prize in Physics in 2010.
- Andre Geim (b. 1958, Sochi, Russia; Dutch citizenship) completed his studies at the Institute of Solid State Physics in Chernogolovka (1987). He currently leads the University of Manchester’s Center for meso-science and nanotechnology. Notably, Geim also won the Ig Nobel Prize in 2000 for levitating a frog using magnetic fields—an experiment conducted with Sir Michael Berry from the University of Bristol.
- Konstantin Novoselov (b. 1974, Nizhny Tagil, Russia; Russian-British citizenship) specialized at Radboud University in Nijmegen, the Netherlands, and now works at the University of Manchester.
Understanding Graphene
Before delving into graphene’s detailed structure and properties, let’s look at it from a simpler perspective. Often hailed as one of the greatest revolutions in new materials for the 21st century, graphene is so strong that a hypothetical “graphene hammock” could support a cat’s weight while weighing less than one of the cat’s whiskers—and remain virtually invisible (Geim & Novoselov, 2007). Since its isolation in 2004, graphene has been identified for millions of potential real-world applications, many of which are already in various stages of research and development.
Extraordinary Strength and Minimal Weight
Graphene is roughly 200 times stronger than steel while being about 1,000 times lighter than a sheet of paper (Lee et al., 2008). Few could have predicted that a material so thin—consisting of a single layer of atoms—could conduct electricity so efficiently and offer such outstanding mechanical resilience. Researchers continue to uncover new properties and potential uses for graphene, ranging from flexible electronics to specialized coatings.
Current Research and Industry Interest
Although many of graphene’s applications are still under development, large corporations have begun integrating or experimenting with graphene. For instance, Toshiba has looked into using graphene-based materials to coat internal cables in electronic devices. In China, prototypes of flexible screens made from graphene have already been demonstrated, and aerospace giants like Airbus and Boeing are evaluating its use for reinforcing aircraft structures.
Moreover, graphene’s potential extends into biology and medicine, where it may be used to create artificial organs with stem cells, develop biosensors, or fabricate advanced drug delivery systems. Such wide-ranging possibilities explain why “the wonder material” continues to captivate both scientific and industrial communities worldwide.
Part 2: Properties of Graphene
Graphene has earned its reputation as the “material of the future” for a reason. It offers a remarkable set of properties—high thermal and electrical conductivity, incredible strength, flexibility, and light weight—all in a single-atom-thick sheet of carbon. In fact, graphene can be about 200 times stronger than steel, with a stiffness comparable to diamond, yet it is lighter and more flexible than carbon fiber.
Key benefits include:
- Low Joule Effect: Graphene produces minimal heat when conducting electricity.
- Low Power Consumption: Consumes less electricity than materials like silicon for similar tasks.
- Transparency: Nearly transparent, absorbing only about 2.3% of visible light.
- High Density: Dense enough to block gases like helium, yet allows water vapor to pass under certain conditions.
- Self-Repair Potential: Early research suggests graphene can self-repair small defects, possibly extending its usable life.
Key Points at a Glance
- Extreme Strength
- Superior Electrical Conductivity
- Exceptional Thermal Conductivity
- Single-Atom Thickness
- High Flexibility
- Lightweight Nature
- Transparency
- Chemical Stability
- Large Surface Area
- Biocompatibility
Why Graphene is Important?
Graphene is often called the “miraculous material” of the 21st century, impressing both scientists and media with its outstanding properties. In particular, its electronic capabilities make it ideal for:
- High-speed transistors for ultrafast circuits
- Potential silicon replacement in microchips, reshaping future computers and devices
The Graphite Factor
High-purity graphite (the raw source of graphene) can be pricey and isn’t always easy to find. Costs range from $2,000 per ton for 97% purity to $20,000 per ton for 99.99% ultrapure graphite. Even so, graphene can be applied in countless ways—from carbon fiber alternatives to advanced electronics—thanks to its incredible versatility. "You could theoretically roll up a graphene-based iPhone and tuck it behind your ear like a pencil,” says Professor James Tour of Rice University, highlighting graphene’s remarkable flexibility.
Future Tech & Investment Possibilities
- Credit Cards could one day have smartphone-level processing power if graphene replaces plastic components.
- European Commission: Investing €1 billion in graphene research over the next decade.
- Samsung & Sungkyunkwan University: Developed a 25-inch flexible graphene touch screen.
- IBM: Created a 150 GHz transistor (current silicon transistors often reach only ~40 GHz).
Despite significant funding and high market expectations, scientists note that manufacturing challenges and cost barriers remain. Once these hurdles are addressed, graphene’s potential could transform consumer devices, computing, and more.
Flexibility & Strength of Graphene
Graphene is incredibly thin yet impressively flexible and robust:
Strength:
- Tensile strength can reach 130 GPa—about 200 times stronger than steel.
- Covalent bonds between carbon atoms make it extremely tough and difficult to break.
Flexibility:
- Can stretch up to 20% of its original length without breaking.
- This flexibility comes from relatively weak interactions between its carbon layers, allowing it to bend and deform without losing integrity.
Because of these traits, graphene is perfect for lightweight aerospace materials, sensors, batteries, medical devices, and flexible electronics (such as bendable screens or bulletproof armor).
Figure 3. Strenght of Graphene
Mechanical Resistance of Graphene
Graphene owes its exceptional mechanical strength to σ-type covalent bonds between carbon atoms. In traditional tensile tests, stress (σ\sigmaσ) is applied to a sample until it breaks, and the resulting strain (ϵ\epsilonϵ) is measured. However, for microscopic membranes like graphene, specialized methods are needed to assess these properties accurately.
Graphene: The Strongest, Hardest & Lightest Material
In two-dimensional form, graphene stands as the strongest material ever discovered. Yet researchers have struggled to leverage this 2D strength in 3D structures. One breakthrough involved compressing small graphene flakes under heat and pressure, creating coral-like shapes with large surface areas and exceptional strength. This implies other strong, lightweight materials could benefit from 3D engineering similar to graphene.
How Strong is Graphene Compared to Other Materials? (Top 10 Strongest Materials Comparison)
When talking about the strongest materials, we can find many competitors of Graphene. Let’s discuss the top ten strongest materials in the world.
1. Diamond
- A naturally occurring carbon crystal, extremely hard (Mohs scale 10).
- Difficult to scratch; high atomic bond density gives it top-tier hardness.
2. Spider Silk
- A biological fiber that can withstand surprising tension relative to its weight.
- Weaker than graphene on a per-weight basis but still stronger than many alloys.
3. Carbon Composites (e.g., Carbon Fiber)
- Consist of carbon filaments bound in a polymer matrix.
- Known for impressive strength, stiffness, and lightness, widely used in aerospace and sports.
4. Osmium
- The densest naturally occurring element in the platinum family.
- Extremely hard in metallic form, yet heavier and less flexible than graphene.
5. Martensitic Steels
- Heat-treated alloys with superior strength compared to standard steel.
- Maintain some malleability, but are heavier than graphene.
6. Wurtzite Boron Nitride (WBN)
- Structurally similar to diamond but about 18% harder.
- Formed under high heat and pressure (e.g., in volcanic eruptions).
7. Lonsdaleíta
- A rare diamond variant, theoretically 58% harder than standard diamond.
- Sometimes forms when meteorites containing graphite impact Earth.
8. Carbon Nanotubes (CNTs)
- Cylindrical, tube-like structures of carbon atoms.
- Known for extraordinary tensile strength and electrical conductivity, yet challenging to produce at large scales.
9. UHMWPE (Ultra-High-Molecular-Weight Polyethylene)
- A thermoplastic with extremely long polymer chains.
- Offers a high strength-to-weight ratio, often used in bulletproof vests.
10. Carbyne
- A 1D chain of carbon atoms with alternating single and triple bonds.
- Predicted to be about twice as strong as graphene, but very unstable and difficult to synthesize in large quantities.
Despite these competitors, graphene (a single atomic layer of carbon arranged in a honeycomb lattice) remains unmatched in tensile strength when measured by thickness. In its perfect crystalline form, graphene can be around 200 times stronger than steel—making it the “king” among the strongest known materials.
Conductivity of Graphene
Conductivity is the ability of a material to transfer sound, electric charge, or heat. In technical terms, it often depends on electron mobility, temperature, and material density or defects.
Research on graphene at the University of Manchester revealed unusual electron behavior, shedding light on new conductivity physics. These insights are critical for designing future nanoelectronic circuits, as graphene’s behavior differs from that of traditional metals.
- Two-Dimensional Lattice: Fewer collisions and scattering events for electrons
- Minimal Imperfections: Leads to higher electron mobility
- Semimetal Properties: Combines metallic conduction with potential insulating characteristics
Electrical Conductivity of Graphene
Graphene showcases incredibly high electrical conductivity, surpassing copper in many conditions. This is primarily due to its semimetal structure, where delocalized electrons freely traverse the lattice, enabling rapid charge flow.
Key Factors
- Purity & Defects: Fewer impurities mean less electron scattering
- Environmental Conditions: Temperature, humidity, and substrate can affect performance
- Intrinsic Lattice Quality: The closer to a perfect 2D crystal, the higher the conductivity
Applications
- Transistors: Smaller, faster switches for high-speed computing
- Flexible Devices: Bendable circuits and sensors that maintain high conductivity
- Energy Storage: Improved electrode materials for batteries and supercapacitors
Thermal Conductivity of Graphene
Graphene also features remarkably high thermal conductivity, with values reaching up to 5000 W/m·K—significantly above most metals and semiconductors. This property could revolutionize thermal insulation and cooling techniques in electronics.
Two Main Reasons
- Strong Covalent Bonds: Efficient heat transfer between closely bonded carbon atoms
- Low Phonon Scattering: Heat-carrying phonons can travel far without hitting defects
Implications
- Thermal Management: Ideal for heat sinks and interface materials in CPUs or other electronics
- Sensors & Actuators: Quick response to temperature changes, enabling precise thermal control
- Innovative Products: Potential breakthroughs in wearable tech, automotive cooling and advanced industrial machinery
Transparency
Graphene is nearly as clear as glass—yet it offers far more exciting possibilities for transparent electronics.
- High Transparency: A single graphene sheet absorbs only about 2.3% of visible light (~97.7% transparency), outperforming conventional glass (around 90%) and plastic (~92%).
- Zero-Bandgap Semiconductor: Electrons in graphene move freely, minimizing light absorption at visible wavelengths.
Influencing Factors:
- Thickness of the graphene film—thicker layers reduce transparency.
- Defects—imperfections cause more light to be absorbed.
- Wavelength—longer wavelengths pass through more easily.
Potential Applications
- Transparent Electrodes for solar cells, touchscreens, and OLED displays.
- Conductive Coatings on windows, offering improved temperature control.
- Thinner, Energy-Efficient Displays that are more responsive than current tech.
Graphene Benchmark in Material Science
Graphene vs. Diamond
Diamond’s three-dimensional carbon lattice gives it extreme hardness (Mohs scale 10), making it highly valued for jewelry and cutting tools. By contrast, graphene arranges carbon atoms in a two-dimensional sheet, offering remarkable tensile strength beyond diamond when measured per atomic layer.
- Key Distinction: Diamond excels in hardness (resistance to scratching), while graphene stands out in in-plane strength.
Graphene vs. Graphite
Graphite consists of multiple graphene layers stacked, concealing graphene’s two-dimensional advantages. When just one layer is isolated, you get a lightweight, highly conductive material with impressive mechanical and thermal properties.
- Key Distinction: Graphite is stable and common, but peeling off a single layer (graphene) reveals world-class strength and conductivity.
Graphene vs. Steel
Steel is an iron-carbon alloy prized for its durability and versatility. However, graphene can be up to 200 times stronger by weight and significantly lighter, opening possibilities for super-strong composites in aerospace, automotive, and more.
- Key Distinction: Steel offers structural reliability, but graphene’s weight-to-strength ratio far surpasses it.
Graphene vs. Carbyne
Carbyne is a one-dimensional carbon chain theorized to be even stronger than graphene, though incredibly difficult to produce at scale. Graphene, on the other hand, is already under substantial research and partial commercialization.
- Key Distinction: Carbyne may lead in theoretical strength, but graphene is more practical given current manufacturing methods.
Graphene vs. Bullet
Graphene has shown impressive ballistic resistance in lab tests, outperforming Kevlar in absorbing projectile impacts. Its lightness and thinness could revolutionize protective gear if large-scale production hurdles are overcome.
- Key Distinction: Graphene’s ballistic potential lies in its ability to disperse energy across an ultra-thin sheet, rather than relying on thickness or weight.
After comparing graphene and other materials, learn how to identify fake graphene.
Part 3: Uses and Applications of Graphene
It has been known for a long time that graphene is the material of the future. More precisely, since 2010, when the Nobel Prize for Physics was awarded to two Russian researchers, Kostya Novoselov and Andre Geim, who, some years before, had succeeded in isolating the thinnest material in the world with the sole aid of a 2-pound scotch roll. Since then, research projects on the material and its possible applications have multiplied all over the world, involving the EU itself, which has earmarked 1 billion euros in the largest European initiative on the subject, Graphene Flagship.
The properties of graphene make it an ideal material for applications in a variety of sectors. For example, graphene can be used in technology, especially in electronics in the manufacturing of integrated circuits. It is assumed that graphene characteristics can make it possible to build processors much faster than current ones.
This speed has already been put into practice in the manufacture of field-effect transistors built with graphene. These transistors also take advantage of the high mobility of carriers with low noise levels presented by graphene.
Among the potential applications of graphene can be cited as the most interesting:
- Ethanol Distillation: Room temperature distillation for fuel and consumption.
- Gas Detection: Ultrasensitive graphene-based gas detectors.
- Optical Modulators: Enhancing light manipulation.
- Solar Cells: Efficient energy conversion.
- Graphene Transistors: Pioneering faster electronics.
- Integrated Circuits: Enhanced speed and efficiency.
- Electrochromic Devices: Advanced light modulation.
- Ultra-Fast Battery Charging: Revolutionizing power storage.
- Transparent Electrodes: For innovative electronic displays.
- Antibacterial Applications: Novel healthcare solutions.
- Radioactive Waste Collection: Streamlining decontamination processes.
- Sports Equipment: Stronger, balanced tools and gear.
- Ultra-Thin Touchscreens: Flexible, durable displays.
- Supercapacitors: Potentially replacing traditional batteries.
- Flash Memory: Faster data storage solutions.
- Biosensors: Quick, efficient health monitoring.
- E-Paper: Updateable and reusable digital paper.
- Audio Technology: Headphones with exceptional sound quality.
- Aerospace and Armor: Enhanced strength and weight efficiency.
- Waterproof Coatings: New levels of protection.
- Bionic Devices: Direct neural integration.
- Folding Batteries: Innovative energy designs.
- Tissue Regeneration: Advancements in medical technology.
- Water Purification: Transforming saltwater into drinking water.
Explore the diverse uses and applications of graphene for further insights.
Graphene's Applications and Real-World Use Cases
Graphene in Composites and Coatings
Graphene is a very thin material, but it has great strength which enhances the mechanical strength of the polymer with high thermal and electrical conductivity such as, 15-50 percent increase in mechanical strength is possible by adding a particular quantity of graphene in nylon 66. When graphene is added in silicone rubber, the tensile strength is enhanced by 2 times for the support component. If graphene is added in nitrile-butadiene rubber, its mechanical properties is enhanced by 1 - 5 times. Also in another normal high polymer like PP, PVC, and resin, the properties of graphene are improved greatly. Graphene's superior properties make it a better material than other coatings in many ways.
Use Case: Graphene 3D's New G6-Impact Composites
The American Graphene 3D Lab has announced the development of a new family of composite materials based on a matrix of crystal polystyrene (HIPS) reinforced with carbon fibers and particles. The products will be marketed under the G6-Impact brand both in the form of a filament for 3D printing and in granules for injection molding. The company produces the materials at its New York facility and is already able to supply limited quantities of application development material.
The performance of these carbon-graphene composites promises to be very high. According to the company, the material offers excellent rigidity combined with extraordinary shock and vibration absorption properties, thanks to both the patent formulation and the production process. The new composites would be suitable for applications requiring high resilience, shock absorption and vibration damping on hard surfaces such as sports equipment, robots, power tool handles car components, drones and parts of airplanes or military equipment.
Graphene in Electronics
Graphene is a material that can revolutionize the composition of electronic devices that we know today. Graphene is so important for every electronic device we use every day.
Thanks to its high electrical and thermal conductivity, Graphene can be widely used in electronics. For example, Transistors, microprocessors and integrated circuits benefit from the mobility of electrons which is particularly high in graphene.
Also for the storage of energy, there may be good developments. The batteries based on graphene, for tablets and other mobile devices, provide performance superior to those current and smaller dimensions because, thanks to its nanometric dimensions, this material can accumulate more energy in smaller spaces.
Use Case: NTNU's Graphene Semiconductors
Semiconductors developed from graphene can change the technology industry in five years. Researchers from the Norwegian University of Science and Technology (NTNU) have developed - and patented - a hybrid material with very interesting properties. These are gallium arsenide nanotubes (GaAs) developed on graphene, which is a layer of highly alloyed carbon atoms arranged in hexagonal order.
According to Professor Helge Weman of NTNU, "The new hybrid material offers excellent optoelectronic properties". He is also the co-founder of CrayoNanoAs, the company created to commercialize the discovery.
The growth of nanowires on graphene occurs with a method called MBE (Molecular Beam Epitaxy). It is a model for a new method of manufacturing semiconductor devices. Among the first future applications, we see solar cells and light-emitting diodes (LEDs).
Companies like IBM and Samsung are leading the development of graphene to replace silicon in electronics and create new applications, such as flexible touchscreens for smartphones. They no longer have to wait. This invention fits perfectly into their production machinery and allows consumer electronics to be brought to a level where design has no limits.
According to the researchers, thanks to this invention in the future we could have self-powered nanomachines and advanced 3D integrated circuits made on graphene and nanowire semiconductors, which allow us to create smaller and more efficient electronics. Furthermore, there is also a discussion of flexible and self-powered consumer electronics to be included in clothes, notepads and other devices, such as smartphones and tablets. Professor Weman highlights that the semiconductors developed on graphene could become the basis of new types of systems and transform the semiconductor industry using graphene as a preferred substrate for many applications.
Graphene in Electrodes
The use of graphene electrodes could lead to the creation of new energy storage devices suitable for electric vehicles, renewable sources and smart grid applications.
In an article published in the prestigious magazine Nano Letters, researchers from two US companies proposed a new technological solution capable of combining the advantages of electrochemical batteries with those of double-layer capacitors (supercapacitors), so to obtain energy storage systems with high power combined with high energy density.
All the scenarios of the possible development of sustainable mobility and energy supply are closely related to the construction of suitable energy storage devices. Currently, the most promising devices are supercapacitors and lithium batteries.
Both solutions have some disadvantages: while lithium batteries offer high energy density with low power density, supercapacitors, on the contrary, can provide high power density, but with low energy density. Supercapacitors, unlike conventional condensers, are not based on the use of a dielectric material. The electrolyte of the condenser, due to the "double layer" electrical effect, leads to effective charge separation, even if the physical separation of the layers is imperceptible.
In general, supercapacitors offer advantages such as high power density, long life, simple recharging circuits, high safety, and low costs. However, they also have some disadvantages, such as a low amount of energy stored per unit of weight, a high self-discharge and a maximum achievable low voltage.
The lithium-ion battery, on the other hand, can generally be described by three main functional components, namely the anode, the cathode and the electrolyte. The non-aqueous electrolyte is commonly made up of a mixture of organic carbonates and contains lithium ions. The cathode is based on metal oxide and the most common material for the anode is graphite. These batteries have the diffusion of lithium ions between the anode and the cathode, with the possibility of these ions to migrate to or from the anode and the cathode. However, the low solid-surface diffusion limits the maximum power density. To date, there is a strong research activity for the improvement of each of these individual devices, but new approaches to the problem are also being investigated.
Use Case: Supercharging Batteries with Graphene Technology
Recently research activities have focused on how to apply nanotechnologies in order to increase the characteristic power density of lithium-ion batteries. In the research presented by Nanotek Instruments and Angstron Materials, the researchers chose a new approach, using nanostructured graphene as electrode material. In prototype devices made during the research, the nanostructured porous graphene is connected both to the anode and to the cathode, in two distinct blocks separated by a porous membrane, and is immersed in the electrolyte. The current flow is based on the exchange of lithium between the surface of the two nanostructured graphene electrodes. The two graphene surfaces can capture lithium ions quickly and reversibly, through surface adsorption mechanisms and/or surface redox reactions.
The authors of the research carried out experiments using different graphene structures. The study is still in a preliminary phase, but results have been so promising that the hypothesis of a future realization of systems able to reach energy densities of 160 Wh/kg per cell unit is possible. This value is over 30 times higher than that achievable with conventional supercapacitors and is comparable with that of lithium-ion batteries. In addition, these systems can reach power densities of 100 kW/kg per cell unit, 10 times higher than those of traditional supercapacitors and even 100 times greater than lithium-ion batteries.
Graphene in LCD and LED Monitors
Graphene is one of the hopes for the achievement of flexible technology. Although today some designs have been achieved based on traditional materials used in electronics, including some timid commercial appearances, the compound that won the Nobel Prize in Physics to Andrey Geim and Konstantin Novoselov promises much more than it has been until now. Another proof of the possibilities of the material are the prototypes of graphene-based LED screens that have been built in the laboratory.
Use Case: Graphene-Driven LED Screens for Next-Gen Electronics
Researchers at the University of Manchester and the University of Sheffield have worked together to develop a semi-transparent device composed of graphene-based LEDs, whose characteristics could be the basis of a future generation of mobile phones, tablets or flexible televisions.
The product resulting from the research is an extremely thin semiconductor (its thickness is between 10 and 40 atoms) that emits light throughout its surface. Scientists have used a combination of graphene with boron nitride and several 2D semiconductors (a structure that also has graphene). The LEDs have been produced at the atomic level.
Graphene-based LED screens are formed by what is called heterostructures. These are the materials resulting from the process of joining different compounds in the form of layers. These heterostructures create a force of attraction for the electrons that give rise to quantum wells, which are used to control the movement of electrons, with which it is possible to favor an exchange that produces photons and emits light. This is the key to the created device to succeed as a screen.
Interesting applications are also those related to lighting, in particular to the realization of LED lamps, more powerful, efficient and durable than the classic ones; a recent discovery conducted by the Graphene Flagship project team also showed, for the first time, the ability of graphene to generate light at the third electrically controllable harmonic: the generation of optical harmonics is a non-linear optical process that creates new colors when a high-intensity laser interacts with a material. In this research, by third-harmonic generation means the production of a light whose frequency is three times that of the incident light. Therefore, starting from the invisible infrared light, intense visible light can be produced.
Graphene in Memories
Graphene is an allotrope of carbon, that is, the property that certain elements have of presenting themselves in different structures, in this case, in a hexagonal tile structure, such as a honeycomb in which each vertex has a carbon atom. Thanks to its properties and the investigations that are being carried out, we are getting to know more applications of this material. In some applications, it could replace or complement silicon as a base material in the world of microelectronics.
Today, electronic devices offer increased storage capacity in a size that is shrinking but, perhaps, current technology is reaching its limits. Precisely, many investigations are exploring new ways to obtain storage systems smaller in size but with a large capacity, for example at the University of California - Los Angeles (UCLA) the research team, together with Samsung, has developed a new type of flash memory that uses graphene along with silicon to store information.
The incorporation of graphene to flash memory could greatly extend the use of this type of technology and open the door to devices with large storage capacity (and a more than manageable size). Currently, chip manufacturers have achieved a high level of integration through the miniaturization of memory cells, nano-sized and based on floating gate transistors.
Use Case: UCLA and Samsung's Graphene-Enhanced Memory for Extended Data Retention
This investigation by UCLA and Samsung would open the door to new miniaturization of memories and, therefore, would extend the life of this type of technology a few more years. Researchers are not removing silicon when using graphene, rather they are using graphene as an aid to extend the range of this technology.
One of the properties of graphene, from the electrical point of view, is that it is a low-consumption material, something that fits perfectly with flash memories and their operating requirements. But, the most important thing about this research is that by adding graphene, it could be said that the memories could store data for 10 years without being lost. In fact, since silicon and graphene-based cells do not interfere with each other, the memories obtained would not present instability problems.
These new generation flash memories made by the research team would consist of cells of about 10 microns in size. The simulation results suggest that devices made with graphene could be miniaturized to 10 nanometers. Current memories begin to be unstable from 22 nanometers. After these results, the team is working on reducing the size of this memory cells to perform new tests and, in addition, thanks to the collaboration with Samsung, it is already in talks with Micron Technology Inc (a chip manufacturer) for the commercialization of this type of new generation memories.
Graphene in Touch Screens
The touchscreens are fashionable in most technology devices, but day by day researchers are trying to find new ways to improve their quality and use. In this mission, a group of researchers from the University of Sungkyunkwan in Korea and Samsung have focused their research on printed graphene, a material made of a carbon sheet with a thickness of a single atom that allows creating flexible screens of great resistance, transparency and with great electrical connectivity.
These researchers have managed to make a layer of graphene of the size of 63 cm, made with the help of a polyester sheet. At present, tin and indium oxide are used as the material for the creation of these screens.
Graphene made of flexible polyester sheets will allow the touch screens to offer great resistance and the possibility of lowering manufacturing costs. According to the researchers, these screens will take time to reach the market since the current manufacturing is based on processes very different from the one proposed.
Use Case: Electronic Circuits Printed Directly on the Clothes
Using conventional inkjet techniques that are economical, safe and environmentally friendly, the researchers recently reported that they had successfully printed 2D materials, creating electronic circuits directly embedded in the fabrics. Researchers have successfully printed graphene directly on fabrics, making it possible to produce wearable integrated electronic circuits by using graphene.
On the subject of flexible and wearable electronic devices, one of the main problems has always been liquid repellency: most electronic circuits do not hold liquids and are therefore not washable (all wearable devices must be first deactivated and removed from clothing before the latter is inserted in the washing machine, with very few exceptions).
And to make matters worse, these are low-cost graphene, printed with techniques similar to inkjet. Only in this case, the ink is made up of graphene flakes, naturally, a stable electric conductor already known. The graphene then undergoes second processing that removes all those non-conductive components.
Learn what is the role of graphene in cutting-edge technologies for daily life.
Graphene in Energy
There are various fields related to energy in which graphene can be incorporated to improve its technology, both in the field of renewable energy and fossil fuel. It is an excellent conductor, better than copper or silver, is chemically inert and is a lightweight material with a contact area due to its shape. It is considered, in turn, a sustainable and non-polluting material. Therefore, graphene's applications to technological reality are multiplying. With the inclusion of graphene in the manufacture of different technological devices some of its applications can be:
- Flexible and transparent touch screens
- Long-lasting batteries: graphene has a greater load capacity
- Cool computers
- Graphene use in Batteries
- More powerful microprocessors, thanks to the fact that graphene loses less energy due to heat, this would increase the speed
- It is a self-repairing material when constituted by being constituted by carbon and hydrogen atoms, and these are found in the air
- Use of Graphene in Solar Cells
- Proton Conductive Membranes
- Transport information faster than fiber optics
- Sensors for cameras with better light sensitivity and greater sharpness
- Ability to absorb solar energy and convert it into useful energy
Graphene in Batteries
Recently, it has been discovered that graphene is able to retain energy more efficiently than graphite. In this way, it is possible to optimize exponentially the recharge of the batteries, in particular, those intended for the power supply of electric vehicles. Through the introduction of some boron atoms, the lithium ions of the batteries are able to adhere more easily to graphene, thus helping to provide an immediate charge. Several manufacturers, such as Hyundai and Kia, have already shown a keen interest in these applications; an example is fuel cell electric cars. Graphene could, therefore, favor the reduction of the cost of hydrogen from renewable sources.
Professor Forsyth of the Faculty of Electrical and Electronic Engineering of the University of Manchester is convinced that the storage of energy through graphene can contribute to increasing the efficiency of electric vehicles, also reducing the weight of the batteries; this would also contribute to further extending the range of electric vehicles. The advantage of using graphene lies in its surface area which is as thin as it is versatile. According to another Manchester professor, Brian Derby, for the material to be actually useful, the layers of atoms must be packaged in a 3D object.
Use Case: Samsung’s Work on Graphene Batteries
Composed of a very thin layer of carbon atoms arranged in a honeycomb pattern, particularly resistant and a perfect electrical conductor, graphene has such great potential that it also arouses the interest of numerous multinationals such as for example, the giant Samsung. In fact, a few months ago it was announced on the blog of the South Korean company that Samsung Advanced Institute of Technology (SAIT), in collaboration with Seoul National University, created a new type of battery using graphene spheres.
Samsung has developed a system capable of transforming graphene, usually produced in two-dimensional sheets a few atoms thick, in small spheres usable as anode and cathode inside the cells of the classic lithium batteries. This three-dimensional structure allows the carbon compound to increase the conductivity of the electrodes and reduce the typical criticality of rechargeable batteries. The technology, in other words, allows to significantly increase the battery yield (+ 45%) and the charging speed (5 times faster), to the advantage also of the recharge times: only 12 minutes compared to the classic batteries in lithium ions that instead take over 60 minutes.
The technology also solves the problem of overheating and dangerous explosions to which storage systems are subjected during recharge, maintaining a constant temperature of 60 degrees Celsius. The study was reported in the journal Nature Communications and is covered by patent filing in South Korea and the United States.
The time to launch the product on the market is quite long, both because the costs of the raw material are still very expensive, and because large-scale production has many technical problems. However, it is easy to understand that Samsung is concentrating its efforts on research and development to create batteries for smartphones, especially after the episodes of overheating and consequent combustion of some Galaxy Note 7 of 2016. The invention is certainly destined to be used to feed the engine of electric cars. The company could, for example, mount its own batteries on Tesla electric cars, ensuring users a range similar to that of petrol cars.
Graphene in Solar Cells
Researchers from the Massachusetts Institute of Technology (MIT) created a new technique in which they use graphene in solar cells, making them transparent, flexible and with the ability to adhere to any type of surface, such as windows, walls and even objects of everyday use, such as cell phones and computers.
Use Case: Kong's Graphene-Based Solar Cells for Integrated Energy Solutions
The device combines graphene electrodes with low-cost organic materials. It is a flexible material, manufactured with carbon sources, cheap and abundant in nature. Professor Jing Kong, from the Department of Electrical Engineering and Computer Science (EECS), one of the authors of the experiment, said he has worked on several alternatives to make flexible solar cells, but so far the best material has been graphene. It is highly conductive, robust, flexible, and transparent.
Kong and his team have conducted tests on various surfaces, such as opaque paper, translucent tape, and plastic. The experiments indicate that the performance of the equipment is similar in the three flexible substrates and lower than those made in glass. They ensure that they can be placed in cars and electronic devices. For example, these new solar cells could be manufactured directly on cell phones and other portable devices instead of being made separately and then installed, a change that would significantly reduce manufacturing costs.
Scientists are now working on improving the efficacy of their organic solar cells based on graphene, without forgoing transparency. They are also considering the best way to expand their solar cells on large surfaces to, for example, cover windows or entire walls, which could generate energy efficiently while remaining imperceptible to the human eye.
Graphene in Proton Conductive Membranes
Fuel cells use hydrogen to produce electricity. The cost of hydrogen, as well as the inefficiency of fuel cells, make this a known but little applicable technology. The incorporation of graphene to the proton conducting membranes that are used in these electric generators could cause the process to increase its efficiency and, therefore, the well-known hydrogen vector acquired the importance or utility that has been given to it in the last decade.
Graphene in Molecular-level Filters
Increasing the efficiency of gas and oil separation from water using graphene sheets is, at the laboratory level, a reality. The strong investments related to this type of technology and the multiple applications (Food, pharmaceutical, oil, and gas industry, so on) where the process could be improved make this application one of the most named in science journals in the recent months. The pressure needed to carry out this process would be drastically reduced compared to reverse osmosis, the most widely used technology to date. The economic war between the proven technologies and the new filters of an atom of thick material is on the table. Graphene could enter, therefore, not as a substitute but as an ally of the processes of the world of oil and gas.
Graphene to Increase Efficiency in Surveys
Some are already considering the possibility of applying graphene for various stages of the processes of exploration and production of crude. Specifically, some studies advance that the use of graphene oxide in surveys would reduce water use by reducing fluid losses to surrounding rocks, compared to the polymers currently used by the drilling industry.
Graphene in Lubricants
A reduction of friction in any process only reduces the energy consumption necessary for it, with the consequent benefits known to all: reduction of the energy bill, reduction of emissions directly or indirectly to the atmosphere, etc. Graphene has been shown to improve as a lubricant, at the laboratory level, to molybdenum or graphite disulfide, behaving in an extraordinary way in humid conditions where the others demonstrate their weaknesses.
Graphene in Biomedical
Known as the "king of new materials," Graphene Nanoparticles not only attracts the attention of society in the fields of electronic products, new energy batteries, and the aerospace industry but is also considered the key to the next transformation in the biomedical field.
Recently, the research team at the University of Olomouc in the Czech Republic announced the use of graphene to develop the smallest metal magnet in the world, which can be used in many fields, such as magnetic resonance, water treatment, biochemistry, and electronics. In view of the larger surface area, biocompatibility and chemical stability of graphene, graphene is highly anticipated in terms of medication administration, cancer treatment and biosensors.
Graphene in Sensors
Graphene has also proved to be very interesting as a chemical sensor: since its composition changes in the presence of gas molecules, this variation can give an estimate of the presence of toxic or polluting gases. Such sensor potential can also be used in medicine. Current studies are adapting graphene to devices for the early diagnosis of hepatitis, a disease that causes more than a million deaths a year worldwide. Similar tests could also be conducted for allergen detection.
In vitro Detection
Biosensing is a new technology that is developing rapidly. In medical applications, the use of graphene as a sensor for in vitro detection is an important direction of research and application.
Research on graphene in the medical field has just begun, some of them remain at the theoretical level and others in the experiment and study. As long as it is expected that the in vitro detection function as a sensor, it will be applied.
The combination of graphene and other nanoparticles as a sensor for in vitro disease detection has been demonstrated in clinical trials. The research team of Shanghai Jiaotong University has made some progress in this regard. They have demonstrated the use of clear breath detection and saliva detection.
There are very few tumor markers or disease markers in exhaled gases, and therefore the sensitivity to detection is extremely high. Compared to traditional routine tests, graphene can improve the sensitivity of detection by at least three orders of magnitude, and some can even achieve the detection of a single molecule.
It is reported that such nanoacupuncture sensing needles are not only stable, but also sensitive to PH, and have excellent detection sensitivity and selectivity for neurotransmitters such as dopamine and can be used for the detection of dopamine in the body.
In addition to in vitro tests, current research also involves graphene for drug delivery vehicles, tumor treatment, antimicrobial sterilization, artificial implant equipment and the like. However, it should not be overlooked that the use of in vivo treatment is still at an early stage and the risk is higher.
Cancer, Biosensors and Nervous Regeneration
A fundamental aspect of the subject in question is that graphene has the ability to interact with living tissue, as highlighted by the professors of the Catalan Institution for Research and Advanced Studies (ICREA), Stephan Roche and Arben Merkoci, in the ICREA Workshop on Graphene Nanobiosensors, organized by the Catalan Institute of Nanoscience and Nanotechnology.
In addition, graphene is compatible with the administration of drugs, cancer therapies or biosensors thanks to its large surface area, biocompatibility and chemical stability.
Nerve tissues and the spine, for example, could be reconstructed thanks to the creation of artificial implants from compounds of highly resistant biological materials based on graphene and their electrical conductivity. In fact, researchers at the Michigan University of Technology are making tremendous advances in the bioprinting of nerves in 3D by developing polymeric materials that can serve as scaffolds for these nerve tissues.
Biosensors is another field open to this technology. Graphene has exceptional performance in the detection of food toxins, environmental pollution, germs, and specific bacteria. Its sensitivity in detecting toxins is 10 times greater than conventional sensors. It can also be used to predict heart attacks by detecting specific microparticles in the blood.
Graphene can be applied to DNA sequencing, which is crucial for studying diseases of genetic origin, types of cancer and problems in immune systems. This membrane-converted material can be immersed in the conductive fluid and apply an energy voltage to extract the DNA through the tiny pores in the graphene (nanopores sequencing). Graphene-based DNA sensors allow faster and more efficient analysis and fall within the scope of nanomedicine. Although the technique is still in its most initial stage, it is an encouragement for researchers on issues such as prevention and treatment of diseases related to genetics.
Graphene in Other Medical Applications
Graphene Nanoparticles can be used for medication administration, mainly due to its great appearance, which promises to administer large quantities of medications to specific areas of the body. Graphene oxide can be used as an anticancer agent against specific cancer cells. Combined with current therapies, it can reduce the tumor size, slow the rate of cancer development and relapse after treatment.
Experiments have shown that graphene can be used as an immune synthesis agent to help other drugs improve the therapeutic effect. The coupling of graphene and sarcosine can stimulate immune cells in the body to secrete more cytokines, while cytokines have a destructive effect on tumors, and immunity obviously improves. But this technology still has some distance to the human body.
Because graphene has good conductivity, it can be modified on the surface of the electrode and inserted inside the nerve, which is useful for the treatment of electrical stimulation, such as senile dementia. Previously, researchers at the Michigan Institute of Technology introduced graphene into 3D-printed neural arrangements and developed a polymer material to foster the arrangements using graphene as an electrical conductor.
Researchers from the University of Cambridge in the United Kingdom have successfully implanted graphene electrodes in the brains of mice and connected them directly to neurons. After conducting experiments, the researchers discovered that electrodes made of graphene material can be safely connected to brain neurons, and after connecting, these neurons can transmit radio signals normally and transmit brain wave signals to the outside world to clear up the outside world.
This technology can be used to repair the sensory function of amputation, paralysis and even Parkinson's disease in the future, to help them recover better, for example, to receive signals from brain waves by means of a robotic arm to capture the action.
Whether it is drug administration or cancer treatment, the use of these treatments remains at the experimental level. The safety problem of graphene for in vivo treatment has been the study site risk. Once it cannot be excreted from the body, it will enter the liver and lungs, causing irritation of the cells and causing injuries.
Graphene in Membranes
Graphene has also shown remarkable applications in important and very fruitful techniques such as desalination which, thanks to a reverse osmosis experiment conducted in the USA allowed water to pass through on one side and salts remaining on the other side of the layer, showed that the peculiar molecular structure of graphene allows creating holes of any size on its surface. Even in cycling, the material finds considerable popularity, being to increase resistance, grip and speed in the mixture of tires and managing to fill the empty space that separates the different rubber molecules, in fact creating a link with them.
Researchers at CiQUS, the Catalan Institute of Nanoscience and Nanotechnology, and the Donostia International Physics Center (DIPC) have successfully synthesized a graphene membrane with pores whose size, shape and density can be modified in the nanoscale with atomic precision. This work opens up the potential of this precious material to applications in electronics, filters and sensors. The results are published in the Journal Science with Dr. Cesar Moreno as the first author, based on a molecule synthesized in the CiQUS by Dr. Manuel Vilas Varela. The work has already resulted in an application for patent on the resulting porous graphene membrane.
The basic features of graphene can be modified due to the presence of pores. This makes it permeable and beneficial as a sieve. This allows it to produce more efficient filters for tiny elements. The pores’ spacing can also be decreased to a few atoms. The use of graphene is possible for replacing the rigid silicon-based and more voluminous elements.
However, while all this is possible in theory, producing a material with these properties requires precision that is not yet within the reach of current manufacturing techniques. The problem is how to approach it: drilling the pores in a material of a thick atom is a task of enormous complexity. Therefore, the researchers adopt in this work a bottom-up strategy, based on the principles of molecular self-assembly and 2D polymerization. They manage to build the graphene mesh with the nanopores already integrated from the beginning.
For this strategy to work, a very exact precursor element is needed, designed to respond to particular stimuli, which will be employed to assemble a large puzzle. In this work, the precursors designed and produced by specialists in synthetic chemistry of the CiQUS were subsequently transferred to ICN2 to be assembled, forming graphene nanopores using the aforementioned bottom-up method. The precursor molecules were subjected to several rounds of heating at high temperatures on a gold surface, which served as a catalyst for the reactions that allowed the molecules to polymerize, forming "nanotyras" of graphene. These structures were then linked laterally, thus obtaining a mesh structure with nanopores of uniform size and spacing.
Simulated in the DIPC and tested in ICN2, the result of this process is a new type of graphene that has electrical properties similar to those of silicon, which can also be used as a highly selective molecular sieve. Together, these two properties point to the development of devices that act simultaneously as a filter and sensor allowing not only the separation of specific molecules but also blocking and / or monitoring the passage of said molecules through the nanopores, using an electric field. This electrical signal would allow obtaining qualitative and quantitative information on which molecules pass at each moment, something that could be applied, for example, in more efficient DNA sequencers.
Thus, the applications of a nanoporous graphene mesh with atomic precision are varied and numerous, ranging from tools to measure and combat the occurrence of contaminating elements to water desalination and also including biomedical applications. In them, a flexible and biocompatible membrane can be employed to recover the function of organs for example the kidney, the natural filter par excellence.
Graphene in Armor
Studies on the ultralight graphene material show that it is an ideal material for bulletproof vests or armor, beating steel and Kevlar. The tests conducted by Rice University and the University of Massachusetts-Amherst give reasons to believe that graphene’s uses are even more far-reaching than previously assumed.
According to anEngadget report, the teams tested the sheets of the material by firing glass bullets of micron size at 6,700 mph, a speed approximately three times faster than an M16 bullet, in graphene layers. The tests showed that graphene was able to better absorb the impact of bullets compared to other materials, deforming in a cone shape before cracking.
While ballistic tests showed that graphene responded well to the impact, scientists still see some problems with its fragile nature, which makes it difficult to form a solid material. The solution, say the scientists, would be to create a compound that combines graphene with something that improves the structure.
According to a report from the Utah People's Post, in addition to improving armor and bulletproof vests, tests showed that the material could also have applications when it comes to batteries and fuel cells because it is permeable to protons. Proton permeability could improve hydrogen fuel cells because it can reduce the risk of fuel leakage.
How Do Bullets Stop Using the Lightest Material Possible?
This question has inspired a series of fascinating advances by materials scientists in the search for an advanced lightweight armor that keeps the foot soldiers agile and the vehicle light. Another one comes from researchers at North Carolina State University, who have developed a new type of bulletproof material comparable in performance to conventional armor, with only half the weight.
The team behind the breakthrough was also responsible for an impressive amount of research in 2015, which led to a new type of light armor with the ability to block X-rays, gamma rays and neutron radiation. A year later, they adopted this approach to produce a high-strength shield capable of stopping bullets in his tracks. Other research groups have had similar success using graphene.
How to Make Graphene Armor?
At the heart of all this is what is known as composite metal foam (CMF). This can be made by bubbling gas through molten metal to make a foamy mixture, which can then be cooled to form a light matrix and embedded with hollow metal spheres. The result is a much lighter material than conventional metals but with comparable resistance.
This time, the team led by Afsaneh Rabiei, a professor of mechanical and aerospace engineering, used this approach to produce a steel CMF embedded with steel spheres. This was placed between a ceramic faceplate and a thin aluminum backplate and shot with bursts of bullets to see how it stood.
The projectiles used were .50 caliber bullets and perforating bullets, which were shot into the armor at speeds between 500 and 885 meters per second (1,640 and 2,900 ft/s). The material was able to absorb 72-75 percent of the kinetic energy of the bullets and 68-78 percent of the piercing bullets. In some of the tests, the bursts left no marks on the backplate.
The team imagines this material making its way in armored vehicles for the army, helping to reduce its weight and, therefore, to improve fuel efficiency. The researchers imagine that with more work, they could produce CMF-based materials with even greater performance.

Contact us to learn more about graphene-based vest Grakor.
Graphene in Improving Engines for Supersonic Airplanes
The Pentagon intends to open a new stage in combustion engines. The Pentagon has allocated three million dollars to Princeton University to develop tiny graphene sheets that, added to the fuel used in the engines of supersonic airplanes, achieve an optimization in their operation and a reduction in consumption and environmental pollution. According to scientists, this development can light the birth of a new era in aircraft combustion engines.
The broad advantages of graphene could also reach high-speed aeronautics. A team of engineers and scientists from Princeton University received a grant from the US Air Force to develop graphene nanoparticles that would be able to optimize the fuel used in supersonic airplanes, allowing them to reach higher speeds and save resources. An advance that would constitute a turning point in the use of combustion engines in aviation.
The financial aid amounts to three million dollars, and was granted to the team led by Ilhan Aksay of the Princeton Department of Chemical Engineering. At the same time, specialists from the Department of Mechanical and Aerospace Engineering of Princeton, from the Pennsylvania State University, the University of Delaware, Stanford University and the University of Maryland are also working on it.
According to Princeton University, the project was selected to study how these tiny particles can be added to the fuel of supersonic aircraft as additives, which could help these aircraft fly faster and make their Diesel engines cleaner and more efficient.
The nanoparticles under study are composed of fragments of molecular carbon sheets (graphene) of few Angstroms thick, and it has been shown that they are capable of collaborating in the ignition and burning of fuels at a higher speed, a characteristic that could give birth to a new generation of combustion engines.
Graphene is a surprising quality material. At the same time, it is the strongest, toughest and hardest material ever created.
Among the main objectives of the team led by Princeton engineers, the full understanding of the process of ignition of fuels with the help of nanoparticles, and the certainty about the type of particles that would work best for the construction of the engines of the future is also important. By fully understanding the process, the chances of obtaining greater efficiency in fuel and engines will be increased.
In this way, specialists think they have a more accurate idea about the improvement and optimization rates that can be achieved in supersonic aircraft fuels. If this advance is achieved, one of the main obstacles to further development of supersonic aircraft would be eliminated.
It is important to remember that in these aircraft the engine must run at extreme speeds and, logically, the fuel must move faster and on a larger scale to meet the engine's requirements. At present, the ignition time and the combustion speed limit the development and optimization of this type of engines, indicated for airplanes that reach very high flight speeds.
Background and Future Applications of Graphene in Fuel Additives
Fuel additives made with tiny graphene particles could make supersonic airplanes fly even faster and their diesel engines will have better efficiency and environmental sustainability protection conditions.
In 2003, specialists Ilhan Aksay and Robert Prud'homme, from Princeton University, developed the first commercially viable technique to create graphene through a chemical process that facilitates its division into ultra-thin individual sheets. Here began the story of this project today aimed at aeronautics.
Only one atom thick, graphene guarantees incredible versatility, which is exacerbated through extremely thin sheet configuration. According to those who apply graphene as the future replacement of silicon in the computer area, it manages to combine the most advanced characteristics of carbon nanotubes and molecular electronics.
The result is molecular carbon sheets with unusual physical and electrical properties. To facilitate these developments, the work of a multidisciplinary team will be required, given the complexity involved in the handling of nanoparticles. If the expected results are achieved, the aviation industry could change greatly in the coming years.
Part 4: How is Graphene Made?
In 1975 Lang et al. achieved the formation of monolayer graphite by thermal decomposition of ethylene in Pt single-crystal substrates. However, the lack of consistency between the properties of said sheets and the fact of not identifying the beneficial applications thereof, made the process not studying extensively.
It was not until 1999 when the research to obtain graphene was resumed, the phase of greatest interest in the production of graphene beginning in 2004. The industrial manufacturing of Graphene by Chemical Vapor Deposition method took place in the year 2010.
Now let’s see how graphene is made.
In recent years, different technologies have been developed for the production of graphene, whose products have differences in both composition, size, and number of layers. Graphene production methods can be divided into two main groups:
Top Down & Bottom-up Methods for the Production of Graphene
Bottom-up methods consist of obtaining graphene from smaller entities, such as molecules. Among these methods is epitaxial growth (CVD on metal and ceramic substrates, thermal decomposition of SiC), chemical synthesis (organic synthesis).
Top-down methods consist of obtaining graphene from an entity that contains it, such as graphite. Among these methods are the micromechanical graphite exfoliation, the liquid phase graphite exfoliation and the chemical exfoliation of graphite materials, based on the intercalation between the layers, subsequent oxidation (not always) and finally its exfoliation.
Using these methods, graphene can be derived either from smaller entities like molecules or from larger structures such as graphite through techniques like epitaxial growth, chemical synthesis, and exfoliation.
Graphene Fabrication
The result of these production methods are Graphenes with different qualities, sizes, and a number of stacked layers, and therefore, with different properties. On the other hand, each method implies an order of magnitude difference in the possibility of industrial production, which leads to a variety in prices also of orders of magnitude. For example, the micromechanical exfoliation method of the scotch tape by Geim and Novoselov is the one that allows obtaining graphene of the highest quality, since it is based on HOPG (High Oriented Pyrolytic Graphite), but industrially it is not viable.
Graphene Production Methods
1. Mechanical Exfoliation Method for Graphene Production
There are different Graphene Production Methods. The most common techniques are based on mechanical exfoliation which consists of applying a force to the surface of highly oriented graphite crystals to detach and unfold the crystalline layers until a single layer is obtained.
A mechanical exfoliation method,universally known as the scotch-tape method, use simple adhesive tape to exfoliate the graphite. The technique consists of placing the surface of a graphite crystal on the adhesive tape, peeling off the tape and peeling some layers of material. The ribbon with the graphite imprint is then folded back on itself and performed several times. Each time, the deposited flakes are divided into increasingly thin layers. At the end of the process, the adherent thin flakes can be transferred simply to an insulating substrate. Mechanical exfoliation is the simplest and most accessible method for isolating graphene flakes about the size of a few square microns, useful for basic research on its properties. Unfortunately, this method is not suitable for industrial production.
2. Solvent Exfoliation for Graphene Production
Solvent exfoliation is the most used technique in the scientific landscape. The chemical and mechanical exfoliation techniques, unlike those of synthesis on substrates (also called bottom-up), consist of the separation of the single graphite planes to obtain the single graphene layer on a large scale; for these reasons, these techniques are called top-down.
The liquid phase exfoliation procedure consists of several operations, each of which can be modified in its operating variables (treatment duration, sonicator power, dispersion concentrations, temperature, pressure) making each procedure different from the other. Generally, dispersions are obtained by treating, in an ultrasonic bath, graphite powder in a solvent, so that the energy supplied by the ultrasounds favors the interleaving of the solvent between the planes of the graphite and the separation between them. The power of the bath, however, has a fundamental role since, if it is too high, it can cause the breaking of graphene sheets, vice versa if it is not sufficiently energetic it cannot cause the exfoliation of the graphitic material. After sonication, a liquid is obtained consisting of a homogeneous phase and a large number of macroscopic aggregates that can be separated through a centrifugation operation.
3. Chemical Vapor Deposition for Graphene Production
The CVD technique (Chemical vapor deposition) allows the growth of the material on metallic substrates, generally of nickel (Ni) or copper (Cu), using a mixture of precursor hydrocarbons, such as methane or ethylene in the presence of H 2 at a temperature in the range of 700 °C - 1000 °C. The growth mechanism involves a first phase in which the carbon (transported by a controlled gaseous flow of hydrocarbons and hydrogen) diffuses into the metal substrate. Subsequently, the system is cooled with the consequent segregation of the carbon on the surface; in the end, by exploiting the chemical etching of the metal substrate, it is possible to detach the graphene plane and transfer it to another surface.

Figure 4. Schematic illustration of the graphene and its derivatives synthesis methods. (a) Mechanical exfoliation (b) liquid phase exfoliation, (c) thermal decomposition, and (d) chemical vapor deposition.
How to Make Graphene with a Blender?
The “recipe” of graphene in a blender emerged after the experiments of Jonathan Coleman, a professor at Trinity College in Dublin, in Ireland, and author of the study published in Nature Materials. Coleman, like many other scientists, was looking for cheap and simple ways to produce tons of clean and good quality graphene scales.
The team led by Coleman was funded by the British firm Thomas Swan. Basically, the scientists employed a kitchen mixer and added:
1. 10 to 25 ml of detergent
2. 500 ml of water
3. 20 to 50 grams of graphite powder, found on the tips of the pencils.
Coleman's "recipe" indicates that everything should be mixed for 10 to 30 minutes. Many flakes of graphene are obtained floating in the water. These scales are the size of microns, each one with the thickness of one nanometer (which corresponds to one billionth of a meter) and 100 nanometers long.
According to Coleman, the emulsion in the blender produces a balance between the surfactant and graphite. In the laboratory, it has been proven to separate graphene in centrifuges, spectrometers and electron microscopes, but the "recipe" in the blender was made at the end of the investigation, like a wink from the scientists. In fact, the central study was conducted in an industrial type, of great proportions.
What Type of Blender Does It Need?
For the experiment a 400-watt high power kitchen mixer was used, although for the main study, they used the industrial equipment, obtaining 5 liters of graphene scales floating in the water.
The idea of this work was to demonstrate how easy it can be to produce this great conductor in industrial quantities. Thomas Swan, who funded the research of Coleman and his team, soon patented the process and built a pilot factory to obtain graphene powder so that it could be mixed with other materials.
Currently, graphene flakes are produced industrially and are even sold in online stores. The problem is that the quality is very variable. Sometimes they are defective or mixed with chemicals that decrease their conductive capacity.
Note: The procedure is not recommended at home. This post is merely illustrative and informative, so the responsibility lies with you.
Graphene from Sugar
At Rice University, Texas, researchers produced graphene from sugar. A team of scientists from the University of Hong Kong is working to obtain graphene from organic waste and similar studies are underway in Europe. Using food waste to obtain useful materials would be a great advantage, also because food waste is the main component of urban waste, which becomes more abundant each year.
Currently, there are many methods, but none is "perfect" since, generally, those that provide high-quality sheets do not allow high production at a "reasonable" price. On the other hand, those that do allow a high production of monoamines, give rise to graphenes with more defects and therefore, with worse electrical properties. This is not necessarily negative since not all applications require the same degree of structural perfection.
The good news in this regard is that scientists are constantly working to make possible the large scale production of Graphene at an affordable rate.
Production Difficulties with Graphene
One of the most important challenges graphene faces, meanwhile, is large-scale production. In this race, there are already alternatives that will try to shade it, such as molybdenite. This competitor is being studied at the Swiss Federal Institute of Technology in Lausanne with satisfactory results to date.
Molybdenite allows to reproduce really small electronic components, solves problems such as the oxidation of the pieces, the loss of energy and the instability of performance in the devices, and also has a resistivity band that gives it the innate ability to conduct current sometimes.
Finally, not only is it possible to produce graphene in the laboratory and to see the individual carbon atoms, it is also possible to modify its structure to change its properties, to make it a "functional" material. Today carbon atoms are already being replaced with silicon atoms in order to create innovative electronic devices, but in the near future we can think of modifying the structure of graphene with molecules of biological interest for the development of new medical therapies.
But for graphene to be used on a large scale, it is necessary to find an economical way of producing it, and there is still work to be done on this. At the moment this material can be obtained by mechanically exfoliating a graphite or graphite oxide surface, but procedures are being studied to extract it from organic material.
Have you heard Nanografi's awarded graphene production method Greengraphene?
Part 5: Popular Graphene Products & Solutions
Graphene has a lot of products that have unique properties and have important applications in a variety of sectors. Let’s discuss a few of its products:
What is a Graphene Sheet?
The graphene sheet is a material with extraordinary properties and multiple applications. It is an ultra-thin sheet of carbon atoms, designed by two Russian researchers, Andre Geim and Kostya Novoselov. Thanks to this discovery, the researchers have received the Nobel Prize for Physics.
Andre Geim and Kostya Novoselov have risen to the headlines with the award of the Nobel Prize for Physics for the discovery of the Graphene sheet. The researchers used the peeling technique which consists of tearing off the layers of carbon with adhesive tape, until only one remain. In this way they succeeded in isolating the thinnest sheet in the world, no one else had previously managed to obtain sheets of the thickness of single atoms.
The single layer of Graphene is known as Graphene Sheet. From the electrical point of view, the Graphene sheet is an excellent conductor and can be "functionalized", to create semiconductors, which makes it suitable for use in microelectronics. Graphene sheet also has good thermal conductivity, which allows its use in cases where it is necessary to quickly extract heat from a source, as in microelectronics itself, since the circuits heat up. Graphene sheet has high elastic and mechanical properties (such as diamond) and for this reason, it can be used individually, or inside other materials to give them high mechanical and elastic properties.
Graphene Sheet Thickness
The thickness of the graphene sheet can be modified according to the requirements. The one that is usually used and offered by Nanografi is 35 micrometers. It is the finest material that can be found in the world. The size of the graphene sheet is 29 x 29 cm and the thermal conductivity is 580 W/mK. Moreover, the density of the graphene sheet is 1.82 g/cm 3. If you are looking for a graphene sheet of specific thickness and other characteristics, let us know and we will provide you exactly what you need. The graphene sheet has the same mechanical strength as Graphene.
How do you make graphene sheets?
The Graphene Sheet is made from graphite, the starting point of the process consists of the stacking of carbon sheets on top of one another and is the form we find in nature. What the two new Nobel Laureates did was to understand their peculiar characteristics in order to obtain them, precisely from graphite, making it available in ultra-thin layers, suitable for a variety of applications. But we understand better what graphene sheet is. It is a thin sheet of carbon atoms, organized in a " honeycomb " structure with very particular properties. So, to obtain the Graphene sheet, the mechanical exfoliation method which is also called the scotch tape method is usually employed. With the help of scotch tape, the thin sheet of graphene is produced at high mechanical strength. There are different types of Graphene sheets. It can be transparent and conductive. The graphene sheets are employed for a variety of purposes.
Graphene Nanotubes
Graphene nanotubes are small rolled sheets of graphite with nanometric diameters and lengths of microns. Graphene nanotubes have excellent electrical, mechanical, optical, chemical, and thermal properties for which they are preferred for increasing several products and also to generate new ones.
Graphene sheets can be rolled up to make Graphene nanotubes. The exceptional thermal, mechanical, electrical, chemical and optical properties of graphene nanotubes allow them to be used in a lot of applications. Such applications can obtain important benefits by incorporating graphene nanotubes. Flat screens used by nanotubes as field emitters, Composite materials reinforced with nanotubes, chemical and biological sensors to detect polluting elements. Generally, sectors such as electronics, materials, sensors, chemistry, biotechnology, mechanics, energy, Scientific and photonic instrumentation could be benefitted by the incorporation of graphene in many of the products.
What is Graphene Oxide?
Study of materials has been fundamental throughout the history for the development of various technologies, for example; burning materials to produce fire, sharpening stones to produce spears or the tips of the arrows for hunting, or even the use of light materials that absorb high impacts to produce automobiles today. This has led the human being to carry out an in-depth study on the materials that surround him, and considering that carbon is one of the elements that can take various allotropic forms, the study of the same has caused a diverse range of materials, among which are graphene, graphene oxide and reduced graphene oxide.
Graphene is one of the most promising materials today since it has very useful properties; such as being a much harder material than a diamond, being an excellent conductor of electrical current and its malleability, the latter is very rare in this material since hard materials are usually very poorly malleable and very fragile. This provides a whole world of applications for this material as in the use of touch screens for cell phones.
On the other hand, reduced graphene oxide is an intermediate point between graphene oxide and graphene, since, with the reduction of graphene oxide, the model becomes much more similar to graphene, having similar properties, however, not being the same material, reduced graphene oxide is a worse electrical conductor than graphene and better than graphene oxide.
Let’s study the properties and some of the applications of graphene oxide and reduced graphene oxide.
Properties of Graphene Oxide
In the study of the materials it is important to know the model that governs the material, since, with this, we can obtain physical, chemical and mechanical properties of the material, giving the opportunity to possible applications for said materials. There are several reasons why this occurs, but the complexity of this material is paramount because it is amorphous, however, graphene oxide can generally be described as a two-dimensional sheet that contains honeycomb-shaped carbon atoms of bee with functional groups of hydroxide and oxygen.
Graphene oxide has some great features for example conductivity, which is dependent on its atomic and chemical structure. In general, graphene oxide films have resistance in a sheet with values of 10 12 Ω⁄sq or greater, this means that this material conducts very easily, which is a poor property for conductors, However, for dielectric materials, it is very useful.
What is Reduced Graphene Oxide?
Graphene oxide (GO) is a two-dimensional material, considered as functionalized graphene with oxygenated groups. It is the main precursor for obtaining reduced graphene oxide, since it is susceptible to being reduced (removal of oxygenated functional groups through physicochemical processes) and/or functionalized to facilitate its interfacial interaction with other materials (polymers, inorganic nanoparticles, etc.) and form composite materials, or by self-assembling sheets to produce GO-based macroscopic materials. These hybrid materials have physicochemical properties superior to those of their individual components and are currently used for applications in water remediation, censorship, catalysis, photovoltaic cells, reinforcement material, biomedicine, etc.
How to produce Reduced Graphene Oxide?
The efficacy of chemical and thermal reduction to produce reduced graphene oxide (rGO) from GO was studied and a method was developed for the direct manufacture of graphene oxide microlistons by self-assembling GO sheets. The results on the chemical reduction of GO demonstrated the reducing and stabilizing nature of citric acid, for which a possible reduction mechanism was proposed.
The thermal reduction is carried out in an argon atmosphere, at temperatures between 200 and 1000 °C, supporting the GO in a copper foil (Cu). In a single step, a hybrid compound formed of CuO 2 or Cu particles covered by rGO supported on a matrix of the same material was obtained. It is important to note that it is possible to control the Cu phase as a function of temperature.
Regarding the self-assembly of the GO sheets, particles are added under acidic conditions to produce micropistons. A mechanism has been proposed to explain the GO self-assembly process in a solid-liquid interface.
Resistant, rigid, transparent, flexible, excellent conductor of electricity and heat. These, in short, are the main properties of Graphene, which has become an element of fundamental importance for research. The easy reproducibility at the industrial level is a further incentive towards experimentation and knowledge of what will probably be the main constituent of many applications of the future.
All these particular features make graphene potentially suitable for different types of applications. In the near future, possible uses could include the production of electrical components such as sensors, batteries, solar cells, touch-screens, integrated circuits, and even high-performance composite materials. Its potential would allow it to be used also in the water desalination process. Further research on graphene is important to know its physical, chemical and mechanical properties. With this information, we can see the possible applications that can be given to some specific material.
What is Graphene Nanoplatelets?
Graphene Nanoplatelets are nanoparticles consisting of short stacks of platelet-shaped Graphene Sheets that are in a planar form. Graphene Nanoplatelets are excellent electrical and thermal conductors as a result of their pure graphitic. It can be used in sensors, supercapacitors, wearable electronics, EMI shielding and more.
What is CVD Graphene?
Chemical Vapor Deposition (CVD) is a chemical process that is widely used in the manufacture of high-quality Graphene, CVD graphene films are highly transparent, cost-effective, conductive and highly scalable.
CVD graphene have wide range of applications. It can be used in catalyst, solar energy, graphene optoelectronics, plasmonic and nanophotonics, graphene semiconductor chips, conductive graphene film, biomaterials and bioelectronic applications.
What is Graphene Dispersion?
Graphene Dispersion product comes with the process of dispersing or suspending Graphene Sheets or flakes evenly in a solvent. Graphene is a highly conductive material, and dispersion products can significantly enhance the electrical and thermal conductivity of composites and coatings. Graphene Dispersion products can increase the surface area of the material, providing more opportunities for chemical interactions and making it suitable for applications such as catalysis and sensing. It is suitable for coatings, energy storage, electronics, optoelectronics and biomedical applications.
What is Boron-doped Graphene?
Boron-doped Graphene is a type of graphene that has been intentionally doped with boron atoms to alter its electronic and chemical properties. Boron doping in graphene can introduce p-type doping, which increases the concentration of holes in the graphene structure and can lead to changes in its electronic properties. This results in increased electrical conductivity, improved catalytic activity, and other unique properties. Boron-doped graphene generally used in energy storage & batteries, sensors, fuel cells and photovoltaic applications.
What is Graphene Aerogel?
Graphene Aerogels are materials that have elasticity and they can easily gain their original form after some compression. In addition, the low density of Graphene Aerogels enables them to be very absorbent. Graphene Aerogels can absorb more than 850 times its own weight. This property means that it could be useful for environmental clean-ups like oil spills. It is widespread for supercapacitors, li-ion batteries, environment purposes and solar cell applications.
What is Graphene Quantum Dots?
Graphene Quantum Dots are nanoparticles with a size of less than 10 nm. They have outstanding properties like exceptional biocompatibility, and electronic and photoluminescence properties with a high surface-to-volume ratio. GQDs can disperse in water very easily. GQDs energy band gaps can be adjusted by varying their size. GQDs photoluminescence can be changed parallelly by tuning the band gap. Graphene quantum dots are generally used in bioimaging, solar cells, LEDs, sensors, supercapacitors & micro-supercapacitors and drug delivery systems.
What is Holey Super Graphene?
Holey Super Graphene known as graphene sheet contains a large number of holes/pores. The locations of the holes are important. The distribution of the holes must be uniform. Holey Super Graphene still holds the properties of graphene but Holey Super Graphene has a higher surface area, ion diffusion capacity, chemical reactivity, and fewer stacked nanosheets than graphene. Thanks to these properties, holey graphene shows better electrochemical performance. It has variety of application areas.
Part 6: Future of Graphene
In essence, while the wonder of graphene offers a glimpse into a revolutionary future, it is through the efforts of pioneering enterprises like Nanografi that this future will be realized. With continued investment, research, and collaboration across sectors, we can expect a world transformed by graphene's integration in the coming years.
To get to know us better and explore our products, visit our website.
Part 7: Frequently Asked Questions (FAQ) About Graphene
What is graphene?
Graphene is a one-atom-thick layer of carbon atoms arranged in a hexagonal lattice. It is the thinnest, strongest, and most conductive material known, making it a revolutionary nanomaterial for various applications.
What makes graphene special?
Graphene is 200 times stronger than steel, lighter than paper, and an excellent conductor of electricity and heat. It is also flexible, transparent, and chemically stable, making it suitable for a wide range of industries.
Who discovered graphene and when?
Graphene was first isolated in 2004 by Andre Geim and Konstantin Novoselov at the University of Manchester. Their work earned them the 2010 Nobel Prize in Physics for their groundbreaking discovery.
How is graphene made?
Graphene can be produced using several methods, including:
- Mechanical exfoliation (Scotch tape method) – The simplest method, used in its initial discovery.
- Chemical vapor deposition (CVD) – A scalable method for high-quality graphene production.
- Liquid-phase exfoliation – Used to produce graphene in bulk for industrial applications.
What are the main applications of graphene?
Graphene is used in various industries, including:
- Electronics: Flexible screens, transistors, and next-generation semiconductors.
- Energy storage: Graphene-based batteries and supercapacitors.
- Medical technology: Biosensors, drug delivery, and antimicrobial coatings.
- Aerospace and automotive: Lightweight composites for stronger, more fuel-efficient materials.
How strong is graphene?
- Graphene is 200 times stronger than steel yet incredibly lightweight. Despite being just one atom thick, it can withstand extreme forces while remaining flexible.
Is graphene conductive?
- Yes, graphene is one of the best electrical conductors, even outperforming copper. Its unique structure allows electrons to move through it at near light speed, making it ideal for high-speed electronics and energy storage.
Can graphene be used in batteries?
- Yes, graphene is being integrated into batteries and supercapacitors to improve charging speed, lifespan, and efficiency. Graphene-enhanced batteries charge much faster than traditional lithium-ion batteries.
What is the difference between graphene and graphite?
- Graphite consists of multiple layers of graphene stacked together. While graphite is used in pencils and lubricants, graphene is a single layer with vastly superior strength, conductivity, and flexibility.
Is graphene expensive to produce?
- The cost of graphene production varies. While high-quality monolayer graphene is still expensive, bulk graphene production methods are reducing costs, making it more commercially viable.
Can graphene replace silicon in electronics?
- Graphene has the potential to replace silicon in transistors and semiconductors due to its superior conductivity. However, challenges such as large-scale production and bandgap limitations need to be addressed before widespread adoption.
Is graphene toxic?
- Graphene is generally considered biocompatible, but its safety depends on particle size, structure, and exposure levels. Research is ongoing to assess long-term effects, especially in medical applications.
What is the future of graphene?
- Graphene is expected to revolutionize electronics, energy storage, medicine, and materials science. Researchers are continuously finding new applications, such as wearable technology, quantum computing, and next-generation composites.
References
- Figure 1. Wikipedia, die freie Enzyklopädie. (2023). Graphen. In Wikipedia. https://de.wikipedia.org/wiki/Graphen
- Figure 2. Nanografi Nano Technology (2023). https://shop.nanografi.com
- Figure 3. Graphene is the strongest material. Phys.org. https://phys.org/news/2008-07-graphene-strongest-...
- Figure 4. Wang, X.-Y.; Narita, A.; Müllen, K. Precision synthesis versus bulk-scale fabrication of graphenes. Nat. Rev. Chem.2017, 2, 0100.
- Bonaccorso, F., Sun, Z., Hasan, T., & Ferrari, A. C. (2012). Graphene photonics and optoelectronics. Nature Photonics, 4(9), 611–622.
- Ferrari, A. C., Bonaccorso, F., Fal'ko, V., Novoselov, K. S., Roche, S., Bøggild, P., ... & Garrido, J. A. (2015). Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale, 7(11), 4598–4810.
- Geim, A. K. (2009). Graphene: Status and prospects. Science, 324(5934), 1530–1534.
- Hernandez, Y., Nicolosi, V., Lotya, M., Blighe, F. M., Sun, Z., De, S., ... & Coleman, J. N. (2008). High-yield production of graphene by liquid-phase exfoliation of graphite. Nature Nanotechnology, 3(9), 563–568.
- Lee, C., Wei, X., Kysar, J. W., & Hone, J. (2008). Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science, 321(5887), 385–388.
- Neto, A. H. C., Guinea, F., Peres, N. M. R., Novoselov, K. S., & Geim, A. K. (2009). The electronic properties of graphene. Reviews of Modern Physics, 81(1), 109–162.
- Nobel Prize. (2010). The Nobel Prize in Physics 2010 – Scientific Background. www.nobelprize.org
- Novoselov, K. S., Geim, A. K., Morozov, S. V., Jiang, D., Zhang, Y., Dubonos, S. V., ... & Firsov, A. A. (2004). Electric field effect in atomically thin carbon films. Science, 306(5696), 666–669.
- Park, H. J., Meyer, J., Roth, S., & Skákalová, V. (2014). Growth and properties of few-layer graphene prepared by chemical vapor deposition. Carbon, 77, 514–523.
- Sun, Z., James, D. K., & Tour, J. M. (2021). Graphene chemistry: Synthesis and manipulation. Advanced Energy Materials, 11(4), 2000455.
This content has been updated March 2025.
Recent Posts
-
Graphene Based Neuromorphic Hardware
Introduction The most significant structural challenge of the digital age is the physical distance b …6th Mar 2026 -
Transistor - Effect MXene Membranes
Introduction Material science is undergoing a revolutionary transition from passive and static compo …27th Feb 2026 -
From Vibration to Information
Introduction Over the past decade, materials science has undergone a fundamental paradigm shift from …20th Feb 2026